Important Questions
Multiple Choice questions-
Question 1.Which transition metal can show highest oxidation state?
(a) Sc
(b) Ti
(c) Os
(d) In
Question 2.Which of the following is not an actinoid?
(a) Thorium
(b) Californium
(c) Uranium
(d) Terbium
Question 3.Which of the following would be diamagnetic?
(a) Cu2+
(b) Ni2+
(c) Cd2+
(d) Ti3+
Question 4.Misch metal is an alloy of
(a) La
(b) Th
(c) Ac
(d) none of these
Question 5.Maximum magnetic moment is shown by
(a) 3d8
(b) 3d7
(c) 3d9
(d) 3d5
Question 6. Maximum oxidation number of manganese is in
(a) K2MnO4
(b) MnO2
(c) KMnO4
(d) Mn2O4
Question 7.Electronic configuration of Fez+ ion is
(a) [Ar] 4s23d4
(b) [Ar] 4s13d5
(c) [Ar] 3d6
(d) [Ar] 3d8
Question 8.Electronic configuration of Cr (Z = 24) is
(a) 3d4 4s2
(b) 3d6 4s0
(c) 3d5 4s1
(d) none of these
Question 9.Increasing order of paramagnetism is
(a) Cu2+ CO2+, Mn2+, Ni2+
(b) CO2+, Cu2+, Mn2+, Ni2+
(c) Cu2+, Ni2+, CO2+, Mn2+
(d) Mn2+, CO2+, Ni2+, Cu2+
Question 10.Copper sulphate dissolves in excess of KCN to give:
(a) [CU(CN)4]3-
(b) [CU(CN)4]2-
(c) CuCN
(d) [Cu(CN2].
Very Short Questions–
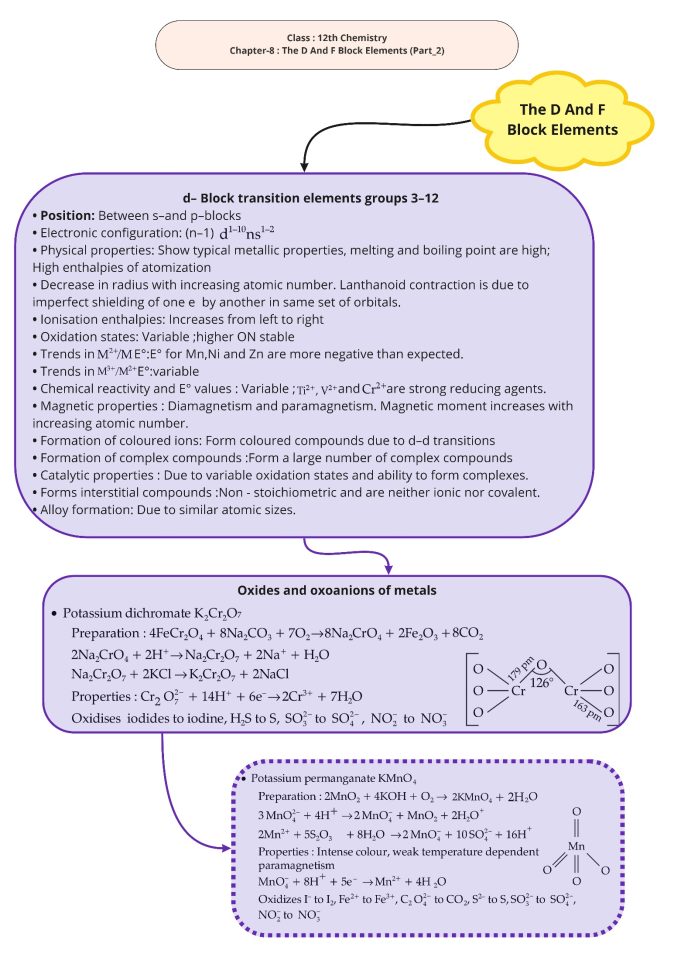
1. Zinc, cadmium and mercury are not considered as transition metals. Why?
2. Write the general configuration of d- block elements.
3. What are the factors that decide the ionization potential?
4. What are interstitial compounds. Give two examples
5. What is the ore of ![]() ?
?
6. What is the effect of adding a base to potassium dichromate?
7. Draw the structure of chromate and dichromate ions?
8. Draw the structure of manganate and permanganate ions?
9. Complete and balance: –
1. ![]()
2. ![]()
3. ![]()
4. ![]()
5. ![]()
6. ![]()
10. Name the two series of f-block.
11. The chemistry of actinoids is more complicated than lanthanoids. Why?
12.What is the general valance configuration of f-block elements?
13. What is the most common oxidation state of lanthanoids and actinoids?
14. Actinoid contraction is more than lanthanoid contraction. Give reason.
15. What is the composition of mischmetal? Give its one use.
16. Actinoids show larger number of oxidation states than lanthanoids. Why?
Short Questions–
1. Give an explanation for the catalytic properties shown by transition metals.
2. Write some characteristics of interstitial compounds.
3. Describe the steps of preparation of![]() ?
?
4. Give some of the uses of![]() ?
?
5. What happens when
(a) A lanthanoid reacts with dill- acid
(b) A lanthanoid reacts with water.
6. Transition metals generally form coloured ions. Why? Which of the following will be coloured? ![]()
7. Explain the steps of preparation of potassium dichromate?
8. What is the lanthanoid contraction? What are its causes and consequences?
Long Questions–
1.Give reasons-
(i) Transition metals have high melting points.
(ii) Second and third transition series have similar radii.
(iii) Second ionization is difficult from Cu and Cr whereas it is easy for Zn.
(iv) Most of the transition elements are paramagnetic.
(v) Transition elements form alloys.
2. Silver atom has completely filled d orbitals ![]() in its ground state. How can you say that it is a transition element?
in its ground state. How can you say that it is a transition element?
3. In the series Sc (Z = 21) to Zn (Z = 30), the enthalpy of atomization of zinc is the lowest, i.e., ![]() . Why?
. Why?
4. Which of the 3d series of the transition metals exhibits the largest number of oxidation states and why?
5. The ![]() value for copper is positive (+0.34V). What is possibly the reason for this? (Hint: consider its high
value for copper is positive (+0.34V). What is possibly the reason for this? (Hint: consider its high ![]() and low
and low ![]() )
)
6. How would you account for the irregular variation of ionization enthalpies (first and second) in the first series of the transition elements?
7. Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
8. Which is a stronger reducing agent ![]() or
or ![]() and why?
and why?
Assertion and Reason Questions–
1.In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Co (IV) is known but Ni (IV) is not.
Reason: Ni (IV) has d6 electronic configuration.
2.In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Transition metals form substitutional alloys.
Reason: Alloys are made to develop some useful properties which are absent in the constituent elements.
Case Study Questions–
1.Read the passage given below and answer the following questions:
The f-block elements are those in which the differentiating electron enters the (n –2)f orbital. There are two series of F-block elements corresponding to filling of 4f and 5f-orbitals. The series of 4f-orbitals is called lanthanides. Lanthanides show different oxidation states depending upon stability of f0, f7 and F14 configurations, though the most conunon oxidation states is +3. There is a regular decrease in size oflanthanides ions with increase in atomic number which is known as lanthanide contraction.
The following questions are multiple choice questions. Choose the most appropriate answer:
2.Read the passage given below and answer the following questions:
The transition elements have incompletely filled d-subshells in their ground state or in any of their oxidation states. The transition elements occupy position in betweens– and p-blocks in groups 3-12 of the Periodic table. Starting from fourth period, transition elements consists of four complete series : Sc to Zn, Y to Cd and La, Hf to Hg and Ac, Rf to Cn. In general, the electronic configuration of outer orbitals of these elements is (n – 1)d1-10 n1-2. The electronic configurations of outer orbitals of Zn, Cd, Hg and Cn are represented by the general formula (n – 1)d10ns2. All the transition elements have typical metallic properties such as high tensile strength, ductility, malleability. Except mercury, which is liquid at room temperature, other transition elements have typical metallic structures. The transition metals and their compounds also exhibit catalytic property and paramagnetic behaviour. Transition metal also forms alloys. An alloy is a blend of metals prepared by mixing the components. Alloys may be homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of the other.
The following questions are multiple choice questions. Choose the most appropriate answer:
MCQ Answers–
Very Short Answers–
Ans 1. Zinc, cadmium and mercury have fully filled ![]() configuration. Therefore, they are not considered as transition metal.
configuration. Therefore, they are not considered as transition metal.
Ans 2. General electronic configuration of d- block elements is![]() .
.
Ans 3.The ionization potential values are governed by various ionization enthalpy values, bond enthalpy values and hydration enthalpy values.
Ans 4. Compounds formed by trapping small atoms like H, C, or N inside the crystal lattices of metals eg. TiC, ![]() etc.
etc.
Ans 5. Potassium dichromate ![]() chromite ore
chromite ore ![]() Potassium Permanganate
Potassium Permanganate![]()
![]() pyrolusite.
pyrolusite.
Ans 6. When a base is added to orange coloured potassium dichromate its colour changes to yellow due to formation of potassium chromate.
![]()
Ans 7.

Ans 8.

Ans 9.
1.![]()
2.![]()
3.![]()
4.![]()
5.![]()
6.![]()
Ans 10.The two series are-
i) 4f series or Lanthanoids
ii) 5f series or actinoids.
Ans 11.The complications in the actinoid series is due to
i)Existence of a wide range of oxidation states.
ii) Their radioactivity.
Ans 12. The general electronic configuration of f-block elements is ![]()
Ans 13. The common oxidation states of
(1) 4f series is +3
(2) 5f series is +3, +4, +5, +6 & +7
Ans 14.The actinoid contraction is more than Lanthanoid contraction as the shielding
Power of 5f orbitals is poorer than 4f orbitals.
Ans 15. Mischmetal is an alloy of a Lanthanoid metal and iron and traces of S, C, Ca &Al. It is used in Magnesium based alloy to produce bullets, shell and lighter Flint.
Ans 16. Actinoids can show many oxidation states as in actinoids 5f orbitals are filled which are not as buried as Lanthanoids and can also participate in bonding to a greater extent besides 6d and 7s electrons.
Short Answers–
Ans 1. Catalytic properties shown by transition metals can be explained due to
(i) Presence of variable valency and ability of elements to form complexes.
(ii) Surface of metals where the reaction can occur.
Ans 2. Some characteristics of interstitial compounds are
(i) They have high melting points.
(ii) They are very hard.
(iii) They retain metallic lustre.
(iv) They are chemically inert.
Ans 3. Potassium Permanganate is prepared in two steps:
Step 1: Fusion of ![]() with KOH and oxidizing agent to give dark green
with KOH and oxidizing agent to give dark green![]() .
.
![]()
Step 2: Disproportionation of manganate ions to give permanganate ions.
![]()
Ans 4. Uses of potassium permanganate –
(a) As an oxidizing agent.
(b) For bleaching of wool, cotton & silk.
(c) For decolourisation of oils.
Ans 5. (i) When a Lanthanoid reacts with dilute- acid, it liberates hydrogen gas.
![]()
(ii) When a Lanthanoid reacts with water, it forms hydroxide.
![]()
Ans 6. Transition metals form coloured ions due to d-d transition. Coloured ions will be those which have unpaired electrons.
|
|
|
Colourless |
|
|
|
Coloured |
|
|
|
Coloured |
|
|
|
Colourless |
|
|
|
Coloured. |
Ans 7. Preparation of potassium dichromate takes place in three steps.
Step 1: Fusion of chromite ore with sodium or potassium carbonate in free excess of air.
![]()
Step 2: Conversion of Sodium Chromate to Sodium Dichromate by acidifying it.
![]()
Step 3: Conversion of sodium dichromate to potassium dichromate by reaching it with KCl.
![]()
Ans 8. Lanthanoid contractions – The cumulative effect of the regular decrease in size or radii of Lanthanoid with increase in atomic number is called Lanthanoid contraction.
Causes – The shape of f orbitals is diffused. They have poor shielding effect due to which the effective nuclear charge increase with increase in atomic number. This causes a decrease in atomic radii
Consequences – Due to Lanthanoid contraction-
1. Radii of the members of the third transition series is similar to those of second transition series.
2. It becomes difficult to separate Lanthanoids.
Long Answers–
Ans.(i) In transition metals besides ns electrons, (n-1)d electrons can also participate in bonding making stronger metallic bonds. This increases their melting points.
(ii) Due to lanthanoid contraction, there is a decrease in size of 5d series. This makes their sizes same as sizes of elements of 4d series.
(iii) In Cr the electronic configuration is ![]() and for Cu, it is
and for Cu, it is![]() . In these after first ionization, which removes the electron from 4s, second ionization requires disturbance in half filled or fully filled configuration which requires high enthalpy whereas the configuration of Zn is
. In these after first ionization, which removes the electron from 4s, second ionization requires disturbance in half filled or fully filled configuration which requires high enthalpy whereas the configuration of Zn is![]() . Here after second ionization, the configuration of Zn is completely filled. Therefore, second ionization is easier for Zn but difficult for Cr and Cu.
. Here after second ionization, the configuration of Zn is completely filled. Therefore, second ionization is easier for Zn but difficult for Cr and Cu.
(iv) Para magnetism in transition elements arises due to presence of one or more unpaired electrons in atomic orbitals.
(v) Due to similarity in their sizes, transition metals can take each others position in their crystal lattice. Therefore they are able to form alloys.
Ans.Ag has a completely filled 4d orbital ![]() in its ground state. Now, silver displays two oxidation states (+1 and +2). In the +1-oxidation state, an electron is removed from the s-orbital. However, in the +2-oxidation state, an electron is removed from the d-orbital. Thus, the d-orbital now becomes incomplete
in its ground state. Now, silver displays two oxidation states (+1 and +2). In the +1-oxidation state, an electron is removed from the s-orbital. However, in the +2-oxidation state, an electron is removed from the d-orbital. Thus, the d-orbital now becomes incomplete ![]() . Hence, it is a transition element.
. Hence, it is a transition element.
Ans.The extent of metallic bonding an element undergoes decides the enthalpy of atomization. The more extensive the metallic bonding of an element, the more will be its enthalpy of atomization. In all transition metals (except Zn, electronic configuration: ![]() ), there are some unpaired electrons that account for their stronger metallic bonding. Due to the absence of these unpaired electrons, the inter-atomic electronic bonding is the weakest in Zn and as a result, it has the least enthalpy of atomization.
), there are some unpaired electrons that account for their stronger metallic bonding. Due to the absence of these unpaired electrons, the inter-atomic electronic bonding is the weakest in Zn and as a result, it has the least enthalpy of atomization.
Ans.Mn (Z = 25) = ![]() Mn has the maximum number of unpaired electrons present in the d-subshell (5 electrons). Hence, Mn exhibits the largest number of oxidation states, ranging from +2 to +7.
Mn has the maximum number of unpaired electrons present in the d-subshell (5 electrons). Hence, Mn exhibits the largest number of oxidation states, ranging from +2 to +7.
Ans.The ![]() value of a metal depends on the energy changes involved in the following:
value of a metal depends on the energy changes involved in the following:
1. Sublimation: The energy required for converting one mole of an atom from the solid state to the gaseous state.
![]()
2. Ionization: The energy required to take out electrons from one mole of atoms in the gaseous state.
![]()
3. Hydration: The energy released when one mole of ions are hydrated.
![]()
Now, copper has a high energy of atomization and low hydration energy. Hence, the ![]() value for copper is positive.
value for copper is positive.
Ans.Ionization enthalpies are found to increase in the given series due to a continuous filling of the inner d-orbitals. The irregular variations of ionization enthalpies can be attributed to the extra stability of configurations such as ![]() . Since these states are exceptionally stable, their ionization enthalpies are very high.
. Since these states are exceptionally stable, their ionization enthalpies are very high.
In case of first ionization energy, Cr has low ionization energy. This is because after losing one electron, it attains the stable configuration ![]() . On the other hand, Zn has exceptionally high first ionization energy as an electron has to be removed from stable and fully-filled orbitals
. On the other hand, Zn has exceptionally high first ionization energy as an electron has to be removed from stable and fully-filled orbitals ![]() .
.
Second ionization energies are higher than the first since it becomes difficult to remove an electron when an electron has already been taken out. Also, elements like Cr and Cu have exceptionally high second ionization energies as after losing the first electron, they have attained the stable configuration (![]() and
and ![]() ). Hence, taking out one electron more from this stable configuration will require a lot of energy.
). Hence, taking out one electron more from this stable configuration will require a lot of energy.
Ans.Both oxide and fluoride ions are highly electronegative and have a very small size. Due to these properties, they are able to oxidize the metal to its highest oxidation state.
Ans.The following reactions are involved when ![]() and
and ![]() act as reducing agents.
act as reducing agents.
![]()
The ![]() value is – 0.41 V and
value is – 0.41 V and ![]() is +0.77 V. This means that
is +0.77 V. This means that ![]() can be easily oxidized to
can be easily oxidized to ![]() , but
, but ![]() does not get oxidized to
does not get oxidized to ![]() easily. Therefore,
easily. Therefore, ![]() is a better reducing agent that
is a better reducing agent that ![]() Fe3+.
Fe3+.
Assertion and Reason Answers–
1. (d) Assertion is wrong statement but reason is correct statement.
Explanation:
Both Co and Ni have (IV) oxidation state. Ni (IV) has 3d6 electronic configuration.
|
Metals |
Outer electronic configuration |
Oxi. states |
|
Co |
3d74s2 |
+2, +3, +4 |
|
Ni |
3d84s2 |
+2, +3, +4 |
2. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
Explanation:
Transition metals form substitutional alloys since they have nearly the same size, they can substitute one another in the crystal lattice.
Case Study Answers–
1. Answer :
Explanation:
Terbium (65), 4f8; Dysprosium (Dy), 4f9; Ytterbium (Yb), 4f13.
Explanation:
The almost identical radii of Zr (160pm) and Hf (159pm), a consequence of lanthanoid contraction.
2. Answer :
Explanation:
The transition metals and their compounds are known for their catalytic activity. This activity is ascribed to their ability to adopt multiple oxidation states to form complexes.
Explanation:
Because of similar radii and other characteristics of transition metals, alloys are readily formed by these metals.
Explanation:
Greater the number of valence electrons, more will be the number of oxidation states exhibited by the element.
Explanation:
All the d-block elements are metals, they exhibit most properties of metals like lustre, malleability, ductility, high density, high melting and boiling point, hardness, conduction of heat and electricity, etc. All the f-block elements are also metals but they are not good conductors of heat and electricity.


