HALOALKANES AND HALOARENES
Based on the structure i.e depending upon the number of halogen atoms in a compound,
Alkyl/ Aryl halides are classified as mono, di, and polyhalogen. When compared to carbon,
halogen atoms are more electronegative. Therefore
Preparation Of Alkyl Halides
Alkyl halides are produced by the free radical halogenation of alkanes-
Step 1 – Adding halogen acids to alkenes
Step 2 – Replacing –OH group of alcohols by halogens with the use of phosphorus halides or
halogen acids or thionyl chloride.
Aryl halides are prepared with the help of electrophilic substitution to arenes. Iodides and
Fluorides are prepared with halogen exchange method. Organohalogens have a higher boiling
point when compared hydrocarbons due to strong van der Waals forces and dipole-dipole
forces. They partial dissolve in water but completely dissolve in organic solvents.
Organometallic compounds are formed by the nucleophilic substitution, elimination, and
reaction with metal atoms which occurs due to the polarity of a carbon-halogen bond of alkyl
halides. Based on the kinetic properties Nucleophilic substitution reactions are classified as
SN1 and SN2. Chirality plays a very important in SN2 reactions of understanding the reaction
mechanisms of these reactions. SN2 reactions are characterized by inversion configuration
whereas SN1 reactions are characterised by racemisation.
Substitution Reaction
The substitution reaction is defined as a reaction in which the functional group of one chemical compound is substituted by another group or it is a reaction which involves the replacement of one atom or a molecule of a compound with another atom or molecule.
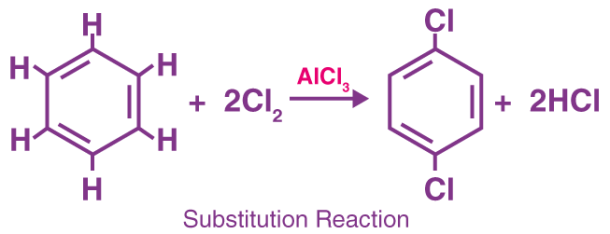
Substitution Reaction Example
These type of reactions are said to possess primary importance in the field of organic chemistry. For example, when CH3Cl is reacted with the hydroxyl ion (OH-), it will lead to the formation of the original molecule called methanol with that hydroxyl ion. The following reaction is as shown below-
CH3Cl + (OH−) → CH3OH(methanol) + Cl–
One more example would be the reaction of Ethanol with the hydrogen iodide which forms iodoethane along with water. The reaction is as shown-
CH3CH2OH + HI → CH3CH2I + H2O
Substitution Reaction Conditions
In order to substitution reaction to occur there are certain conditions that have to be used. They are-
Substitution Reactions – Types
Substitution Reactions are of two types naming nucleophilic reaction and electrophilic reactions. These two types of reactions mainly differ in the kind of atom which is attached to its original molecule. In the nucleophilic reactions the atom is said to be electron-rich species, whereas, in the electrophilic reaction, the atom is an electron-deficient species. A brief explanation of the two types of reactions is as given below.
nucleophiles
Nucleophiles are those species in the form of an ion or a molecule which are strongly attached to the region of a positive charge. These are said to be fully charged or have negative ions present on a molecule. The common examples of nucleophiles are cyanide ions, water, hydroxide ions, and ammonia.
Nucleophilic substitution reaction
A Nucleophilic substitution reaction in organic chemistry is a type of reaction where a nucleophile gets attached to the positive charged atoms or molecules of the other substance.
Nomenclature Of Haloalkanes And Haloarenes
Initially, there was no proper system for the naming of compounds. Mostly there were trivial names that were used depending upon the country and region. These trivial names were based on the discoverer or the nature of the compound or its place of discovery.
The system of trivial names was not standard and led to much confusion, thus raising the need for a standard system for the naming of organic compounds. IUPAC came up with a set of rules that are used universally for the naming of organic compounds.
There are two names associated with every compound:
Common name – It is different from a trivial name in the sense that it also follows a rule for its nomenclature.
IUPAC name – The IUPAC (International Union of Pure and Applied Chemistry) naming system is the standard naming system that chemists generally use.
Rules of Nomenclature
The Methodology of Writing Name
Nomenclature of Haloalkanes
Alkyl halides are named in two ways. In the common system, the alkyl group is named first followed by an appropriate word chloride, bromide, etc. The common name of an alkyl halide is always written as two separate words. In the IUPAC system, alkyl halides are named as haloalkanes. The other rules followed in naming compounds is that
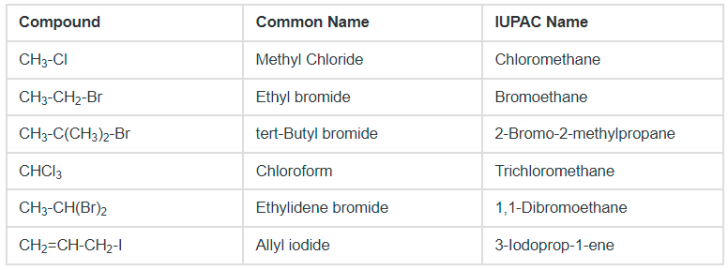
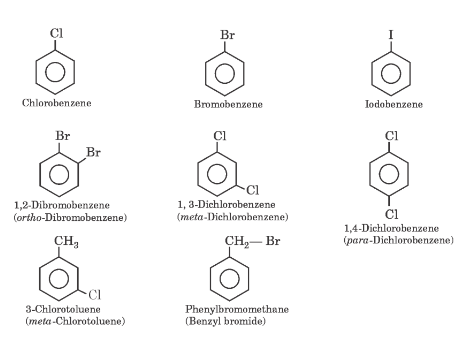
Nomenclature of Haloarenes
The common and IUPAC names of some representative haloarenes are given below.
Haloarenes: Nature of C-X bond
Haloarenes are the chemical compounds containing arenes, where one or more hydrogen atoms bonded to an aromatic ring are replaced with halogens. The nature of C-X bond depends on both the nature of carbon in the aromatic ring and the halogen attached. Halogens are generally denoted by “X”.
As we know halogens are group 17 elements having high electronegativity namely, fluorine (F), chlorine (Cl), bromine (Br), iodine (I) and astatine (At). Out of them, Fluorine has the highest electronegativity. The elements in this group are just one electron short of completing their nearest noble gas configuration.
Carbon in haloarenes is a 14th group element with comparatively lesser electronegativity in comparison to halogen molecules. This is due to the fact that electronegativity increase across a period from left to right.
Salient Points on the Nature of C-X Bond in Haloarenes are:
SN1 and SN2 Reaction of Haloalkanes
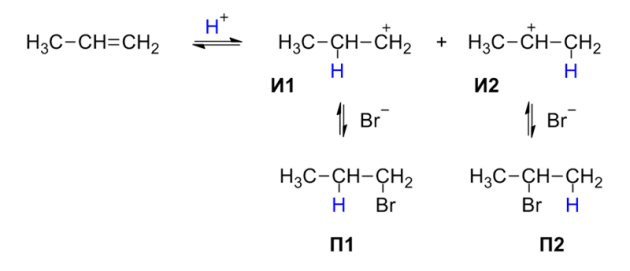
Haloalkanes are converted into alcohols using hydroxide ion in aqueous media through SN1 and SN2 Reactions. Alcohols can efficiently be prepared by substitution of haloalkanes and sulfonic esters with good leaving groups. The choice of reagents and reaction conditions for the hydrolysis is important because competitive elimination reactions are possible especially at high temperatures leading to alkenes.
The hydrolysis of haloalkanes depends on the structure of the haloalkanes, primary haloalkanes typically undergo SN2 reactions whereas tertiary haloalkanes react an SN1mechanism for tertiary haloalkanes or tertiary alkyl halides. There are two kinds of reactions of haloalkanes naming SN1 And SN2 Reaction.
SN1 Reaction
The SN1 reaction is a substitution nucleophilic unimolecular reaction. It is a two-step reaction. In the first step, The carbon-halogen bond breaks heterolytically with the halogen retaining the previously shared pair of electrons. In the second step, the nucleophile reacts rapidly with the carbocation that was formed in the first step.
This reaction is carried out in polar protic solvents such as water, alcohol, acetic acid etc. This reaction follows first order kinetics. Hence, this is named as substitution nucleophilic unimolecular. This reaction takes place in two steps as described below.
Step-1:
Step-2:
SN2 Reaction
This reaction follows second order kinetics and the rate of reaction depends upon both haloalkane and participating nucleophile. Hence, this reaction is known as substitution nucleophilic bimolecular reaction. In this reaction, the nucleophile attacks the positively charged carbon and the halogen leaves the group.
It is a one-step reaction. Both the formation of carbocation and exiting of halogen take place simultaneously. In this process, unlike the SN1 mechanism, the inversion of configuration is observed. Since this reaction requires the approach of the nucleophile to the carbon bearing the leaving group, the presence of bulky substituents on or near the carbon atom has a dramatic inhibiting effect.
So opposite to SN1 reaction mechanism, this is favoured mostly by primary carbon, then secondary carbon and then tertiary carbon. Nucleophilic substitution reaction depends on a number of factors. Some important factors include.
Comparing SN1 and SN2 Reactions
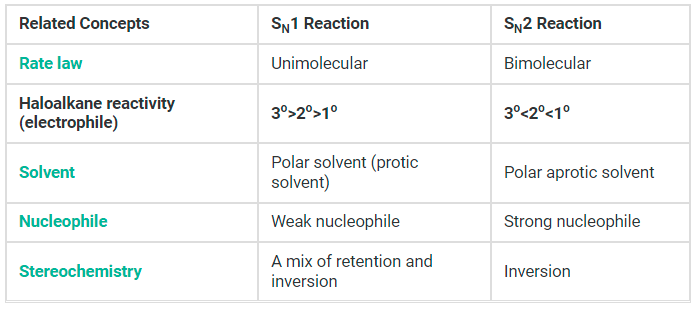
The solvent in which the nucleophilic substitution reaction is carried out also has an influence on whether an SN2 or an SN1 reaction will predominate. Before understanding how a solvent favours one reaction over another we must understand how solvents stabilize organic molecules.
Polyhalogen Compounds

Polyhalogen compounds: Carbon compounds containing more than one halogen atom permolecule.
Polyhalogen compounds are useful in various industries and in griculture.Someimportant polyhalogen compounds:
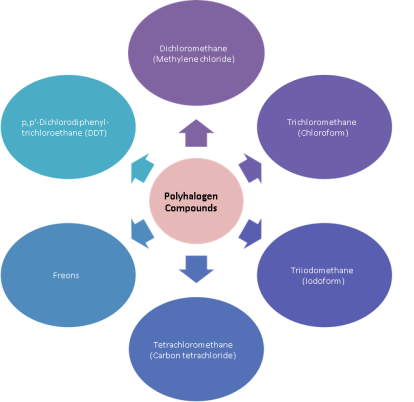
Dichloromethane (Methylene chloride)
Uses:
Dichloromethane(methylenechloride)isusedasa:
Harmfuleffects:
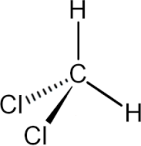
Trichloromethane(Chloroform)
Uses:
Harmfuleffects:
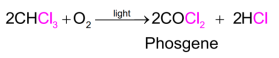
Itisthereforestoredincloseddark-colouredbottleswhicharecompletelyfilledsothatairiskeptout.
Triiodomethane(Iodoform)
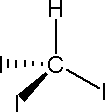
Uses:
Drawback:
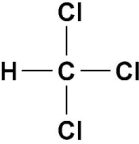
Tetrachloromethane(Carbontetrachloride)
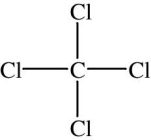
Uses:
Harmfuleffects:
Freons
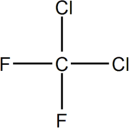
Uses:
HarmfulEffect:
p,p′-Dichlorodiphenyltrichloroethane(DDT)
![]()
DDT,the firstchlorinatedorganic insecticide, wasoriginally preparedin1873.
However,itwasnotuntil1939thatPaulMullerofGeigyPharmaceuticalsinSwitzerlanddiscoveredtheeffectiveness ofDDTasan insecticide.
PaulMullerwas awardedthe NobelPrize inMedicine andPhysiologyin 1948for thisdiscovery.

PaulMuller
Uses:
HarmfulEffects:
Problemsrelatedto extensiveuseof DDT began to appear inthe late1940s.
TheuseofDDTwasbannedintheUnitedStatesin1973,althoughitisstillinuseinsomeotherpartsofthe world.
SN1 mechanism

The tertiary alkyl halides react by SN1 mechanism via formation of carbocation as intermediate. The reactivity order for SN1 reaction is
Benzyl > Allyl > 3°> 2°> 1°> CH3X.
A mechanism for the reaction of tert-butyl chloride with water apparently involves two steps:
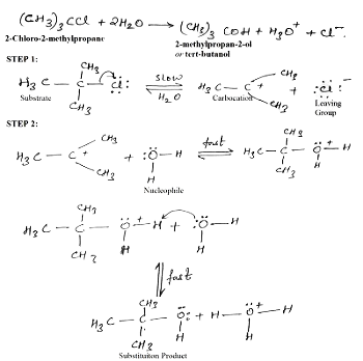
Stereochemistry of SN1:
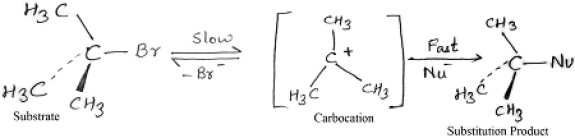
Key features:
More stable will be the carbocation intermediate; faster will be the SN1 mechanism.
Polar solvents lead to polar transition state which in turn accelerates the SN1 reaction.
If the initial compound is chiral then SN1 reaction ends up with racemization of the product.
Weaker bases being leaving group favor SN1 reaction.
SN2 mechanism
In case of SN2 reactions the halide ion leaves from the front side whereas the nucleophiles attacks from the back side; due to this reason SN2 reactions are always accompanied by the inversion of configuration. Thus formation of another enantiomer is lead by SN2 reaction of an optically active halide i.e. optical activity is retained but with opposite configuration.

Stereochemistry of SN2:

Key features:
In SN2 reaction the stereochemistry around carbon atom of the substrate undergoes inversion and is known as walden inversion.
The rate of reaction depends on the steric bulk of the alkyl group.
Increase in the length of alkyl group decreases the rate of reaction. Alkyl branching next to the leaving group decreases the rate drastically.
Under the following conditions SN1 and SN2 reactions take place:
E1 reaction
It is a unimolecular reaction. Rate determining step consist of formation of carbocation intermediate. Stability of carbocation intermediate determines the reactivity of E1 reaction.
Order of reactivity for E1 reaction is 30°> 20°>10°. Both elimination and substitution reaction involves the use of (same reactive intermediate) carbocation. Therefore both the products are formed in comparable amount. This reaction is favored by entropy of reaction therefore increase in temperature favors the E1 reaction.
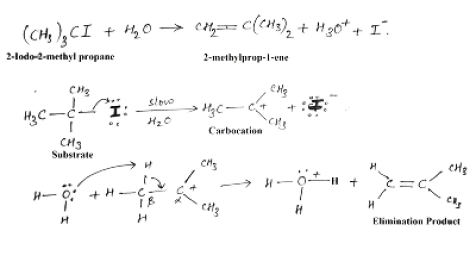
Stereochemistry of E1 reaction:
E1 eliminations generally lead to the more stable stereochemistry.
The rate of the E1 reaction depends only on the substrate, therefore more stable the carbocation is, faster will be the reaction. Slowest step is the formation of the carbocation. Alkenes formation doesn’t require strong base, since there is no leaving group that needs to be displaced. So there is no requirement for the stereochemistry of the starting material;
E2 reaction
It’s a biomolecular reaction. It is a single step reaction whose rate depends on the concentration of base and substrate. Reactivity depends on both strength of base and nature of alkyl halide. Order of reactivity for E1 reaction is 30°>20°>10°. This reaction proceeds at room temperature.
Example:
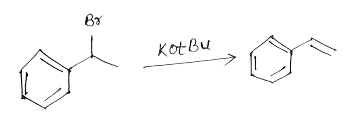
General mechanism:
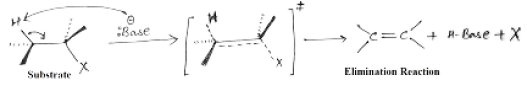
Stereochemistry of E2 reaction:
E2 eliminations may or may not lead to the more stable stereochemistry. Initial material for this reaction has two sp3 hybridized carbons which on rehybridization forms two sp2 hybridized carbons. The C-X bond and the C-H bond lines up in the same plane and faces in anti directions to each other.
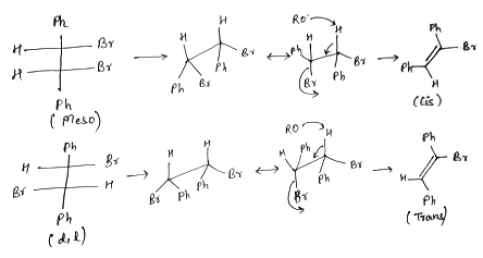
Important Questions
Multiple Choice questions-
1. SN1 reaction of alkyl halides lead to
(a) Retention of configuration
(b) Racemisation
(c) Inversion of configuration
(d) None of these
2. p-djchlorobenzene has higher melting point than its o- and m- isomers because
(a) p-dichlorobenzene is more polar than o- and m- isomer.
(b) p-isomer has a symmetrical crystalline structure.
(c) boiling point of p-isomer is more than o- and m-isomer.
(d) All of these are correct reasons.
3. Chloropicrin is formed by the reaction of
(a) steam on carbon tetrachloride.
(b) nitric acid on chlorobenzene.
(c) chlorine on picric acid.
(d) nitric acid on chloroform.
4. Fitting reaction can be used to prepare
(a) Toluene
(b) Acetophenon
(c) Diphenyl
(d) Chlorobenzene
5. Identify the end product (C) in the following sequence:
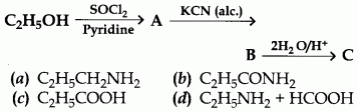
6.
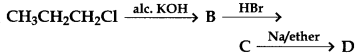
In the above reaction, the product D is
(a) Propane
(b) 2, 3-Dimethylbutane
(c) Hexane
(d) Allyl bromide
7. Identify X and Y in the following sequence
![]()
(a) X = KCN, Y = LiAlH4
(b) X = KCN, Y = H3O+
(c) X = CH3Cl, Y = AlCl3 HCl
(d) X = CH3NH2, Y = HNO2
8. In the following sequence of reactions:
![]()
(a) n-propylamine
(b) isopropylamine
(c) ethylamine
(d) ethylmethylamine
9.
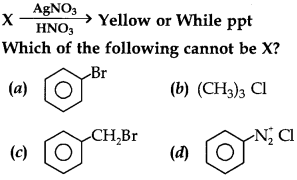
10.
Identifay Z in the series
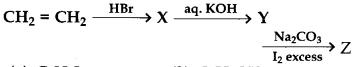
(a) C2H5I
(b) C2H5OH
(c) CHI3
(d) CH3CHO
Very Short Questions-
1. Give IUPAC names of following compounds
(i).
![]()
(ii).
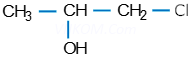
(iii).
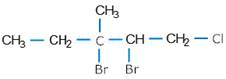
(iv).
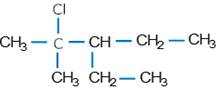
(v).
![]()
(vi).
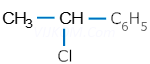
(vii). ![]()
(viii).

(ix).

(x).
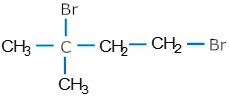
Short Questions-
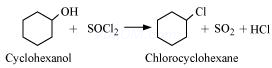
1. Thionyl chloride is preferred for converting alcohol to haloalkane.
2. Phenol cannot be converted to chlorobenzene by reacting with HCl.
3. ![]() is added during iodination of benzene.
is added during iodination of benzene.
4. p- dichlorobenzene has higher melting point than meta – dichlorobenzene.
5. The boiling points of isomeric haloalkenes decrease with increase in branching.
6. Hydrolysis of optically active 2- bromobutane forms optically inactive
butan – 2 – ol.
7. Chlorobenzene is less reactive towards nucleophilic substitution reaction.
8. Chloroform is stored in dark coloured bottles.
9. The order of boiling points is RCl < RBr < RI.
10. Vinyl chloride is less reactive than allyl chloride.
Long Questions-
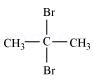
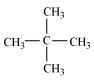
1. Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene
2. Write structures of different dihalogen derivatives of propane.
3. Among the isomeric alkanes of molecular formula![]() , identify the one that on photochemical chlorination yields
, identify the one that on photochemical chlorination yields
(i) A single monochloride.
(ii) Three isomeric monochlorides.
(iii) Four isomeric monochlorides.
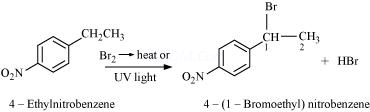
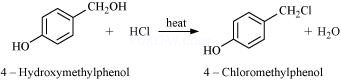
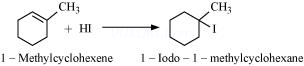
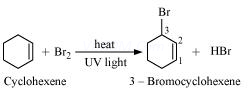
4. Draw the structures of major monohalo products in each of the following reactions:
(i)
![]()
(ii)
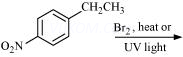
(iii)
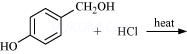
(iv)
![]()
(v)
![]()
(vi)
![]()
5. Arrange each set of compounds in order of increasing boiling points.
(i) Bromomethane, Bromoform, Chloromethane, Dibromomethane.
(ii) 1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
6. Which alkyl halide from the following pairs would you expect to react more rapidly by an ![]() mechanism? Explain your answer.
mechanism? Explain your answer.
(i)
![]()
(ii)
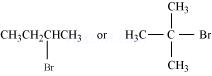
(iii)
![]()
7. In the following pairs of halogen compounds, which compound undergoes faster ![]() reaction?
reaction?
(i)
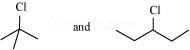
(ii)
![]()
8. Identify A, B, C, D, E, R and ![]() in the following:
in the following:
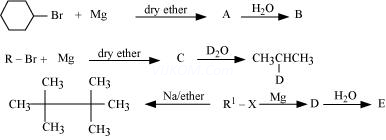
Assertion and Reason Questions-
1. In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Isopropyl chloride is less reactive than CH3 Br in SN2 reactions.
Reason: SN2 reactions are always accompanied by inversion of configuration.
2. In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Lower members of alkyl halides are colourless gases.
Reason: Alkyl iodides in general turn black on exposure to air and light.
Case Study Questions-
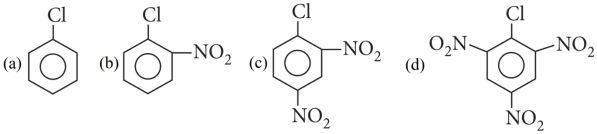
1. Read the passage given below and answer the following questions:
Haloarenes are less reactive than haloalkanes. The low reactivity of haloarenes can be attributed to:
Reactivity of haloarenes can be increased or decreased by the presence of certain groups at certain positions for example, nitro (-NO2) group at o/ p positions increases the reactivity of haloarenes towards nucleophilc substitution reactions.
The following questions are multiple choice questions Choose the most appropriate answer:
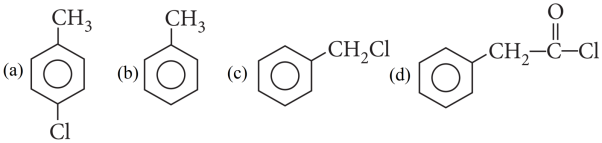
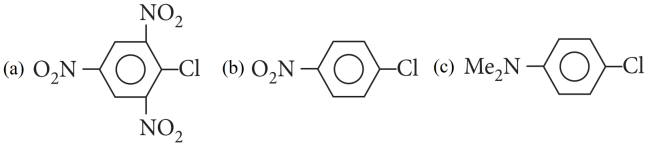
2. Read the passage given below and answer the following questions:
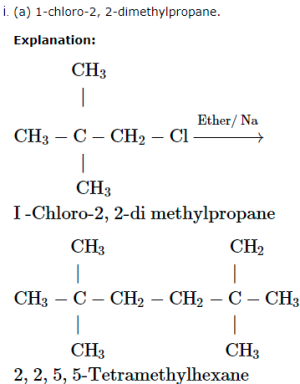
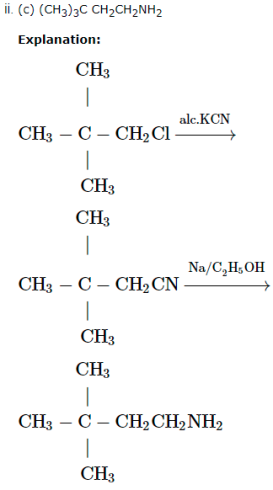
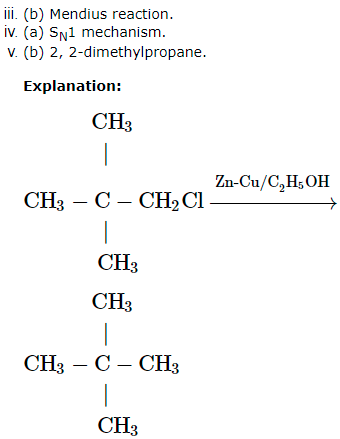
A chlorocompound (A) on reduction with Zn-Cu and ethanol gives the hydrocarbon (B) with five carbon atoms. When (A) is dissolved in dry ether and treated with sodium metal it gave 2, 2, 5, 5 – tetramethylhexane. The treatment of (A) with alcoholic KCN gives compound ( C).
The following questions are multiple choice questions. Choose the most appropriate answer:
MCQ Answers-
Very Short Answers-
(i) 1, 3- Dibromobutane
(ii). 1- Cholopropan-2-ol
(iii). 2, 3 – Dibromo-1-chloro-3-methylpentane
(iv). 2-Choloro-3-ethyl-2-methyl pentane
(v). 1-Chloro-2-phenylethane
(vi). 1-Chloro-1-phenyl ethane
(vii). 1, 2, 3, 4, 5, 6- hexachlorocyclohexane
(viii). 2, 2- Dihexyl 1, 1, 1-Trichloro ethane
(ix). 4, 4-dibromobiphenyl
(x). 1, 3-Dibromo -3- methyl butane
Short Answers-
Ans 1. Thionyl chloride is preferred for converting alcohol to haloalkane because the bi- products formed are all gases which escape into the atmosphere.
![]()
Ans 2. In phenol, due to resonance, the carbon –oxygen bond has a partial double bond character and is difficult to break being stronger than a single bond. Therefore, it can-not be converted to chlorobenzene by reacting with HCl.
Ans 3. When benzene is reacted with iodine, the reaction is reversible in nature. It leads to the formation of reactants back. Therefore, and oxidizing agent like ![]() oxidizes the HI formed in the reaction and keeps the reaction in forward direction.
oxidizes the HI formed in the reaction and keeps the reaction in forward direction.
Ans 4.
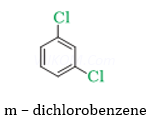
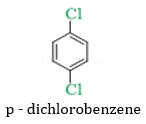
p- dichlorobenzene is having symmetrical structure therefore it can fit better into the crystal lattice which increases its melting point.
Ans 5. The boiling points of isomeric haloalkanes decreases with branching due to decrease in surface areas with branching. As branching increasing the structure becomes more spherical and the surface area decreases. e.g. the boiling points of isomers of ![]() Br follows the order.
Br follows the order.
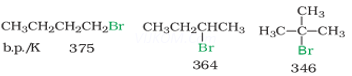
Ans 6.
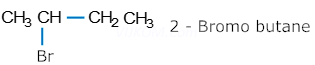
The compound undergoes hydrolysis by SN1 mechanism via the formation of carbocation which is planar.
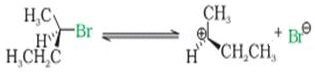
The attack of nucleophile can result in product which is a mixture of compounds both with same configuration and inverted configuration.
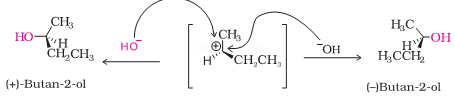
Therefore it results in the formation of racemic mixture which is optically inactive.
Ans 7. Chlorobenzene is less reactive towards nucleophilic substitution due to –
i. resonance, C- Cl bond acquires a double bond character and becomes stronger than a single bond.
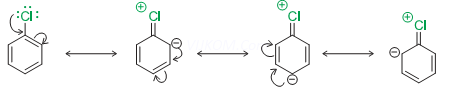
ii. ![]() hybridisation in C of C-X bond, the carbon becomes more electronegative and holds the electron pair of C-X bond more tightly decreasing the bond length.
hybridisation in C of C-X bond, the carbon becomes more electronegative and holds the electron pair of C-X bond more tightly decreasing the bond length.
iii. Instability of phenyl cation.
iv. Repulsion for incoming nucleophile from electron rich ring.
Ans 8. Chloroform gets oxidsed slowly by air in the presence of light to an extremely poisonous gas phosgene. Therefore, to avoid any exposure to air and sunlight, it is kept in dark coloured bottles.
![]()
Ans 9. The boiling points of alkyl halides depends on dipole and van-der-waal’s interaction. These attractions get stronger as the molecules get bigger in size and have more electrons. As the size of halogens increases in the order –
![]()
The boiling points also follow the order
RCl < RBr <RI
Ans 10. Due to resonance C- Cl bond gets double bond character and becomes stronger than a single bond, making vinyl chloride less reactive than allyl chloride.
Long Answers-
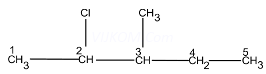
Ans 1. (i) 2-Chloro-3-methyl pentane
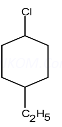
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4- tert-Butyl-3-iodoheptane
(iv) 1, 4-Dibromobut-2-ene
![]()
(v) 1-Bromo-4-sec-butyl-2-methylbenzene

Ans 2. There are four different dihalogen derivatives of propane. The structures of these derivatives are shown below.
(i) 1, 1-Dibromopropane
![]()
(ii) 2, 2-Dibromopropane
(iii) 1, 2-Dibromopropane
![]()
(iv) 1, 3-Dibromopropane
![]()
Ans 3. (i) To have a single monochloride, there should be only one type of H-atom in the isomer of the alkane of the molecular formula ![]() . This is because, replacement of any H-atom leads to the formation of the same product. The isomer is neopentane.
. This is because, replacement of any H-atom leads to the formation of the same product. The isomer is neopentane.
Neopentane
(ii) To have three isomeric monochlorides, the isomer of the alkane of the molecular formula ![]() should contain three different types of H-atoms.
should contain three different types of H-atoms.
Therefore, the isomer is n-pentane. It can be observed that there are three types of H atoms labelled as a, b and c in n-pentane.
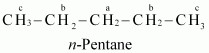
(iii) To have four isomeric monochlorides, the isomer of the alkane of the molecular formula C5H12should contain four different types of H-atoms. Therefore, the isomer is 2-methylbutane. It can be observed that there are four types of H-atoms labelled as a, b, c, and d in 2-methylbutane.
Ans 4. (i)
(ii)
(iii)
(iv)
(v)
![]()
(vi)
Ans 5. (i)

For alkyl halides containing the same alkyl group, the boiling point increases with an increase in the atomic mass of the halogen atom.
Since the atomic mass of Br is greater than that of Cl, the boiling point of bromomethane is higher than that of chloromethane.
Further, for alkyl halides containing the same alkyl group, the boiling point increases with an increase in the number of halides. Therefore, the boiling point of Dibromomethane is higher than that of chloromethane and bromomethane, but lower than that of bromoform.
Hence, the given set of compounds can be arranged in the order of their increasing boiling points as:
Chloromethane < Bromomethane < Dibromomethane < Bromoform.
(ii)
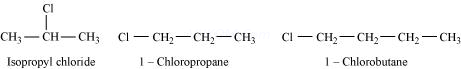
For alkyl halides containing the same halide, the boiling point increases with an increase in the size of the alkyl group. Thus, the boiling point of 1-chlorobutane is higher than that of isopropyl chloride and 1-chloropropane.
Further, the boiling point decreases with an increase in branching in the chain. Thus, the boiling point of isopropyl alcohol is lower than that of 1-chloropropane.
Hence, the given set of compounds can be arranged in the increasing order of their boiling points as:
Isopropyl chloride < 1-Chloropropane < 1-Chlorobutane
Ans 6. (i)

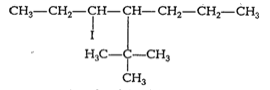
2-bromobutane is a ![]() alkylhalide whereas 1-bromobutane is a
alkylhalide whereas 1-bromobutane is a ![]() alkyl halide. The approaching of nucleophile is more hindered in 2-bromobutane than in 1-bromobutane. Therefore, 1-bromobutane reacts more rapidly than 2-bromobutane by an
alkyl halide. The approaching of nucleophile is more hindered in 2-bromobutane than in 1-bromobutane. Therefore, 1-bromobutane reacts more rapidly than 2-bromobutane by an![]() mechanism.
mechanism.
(ii)
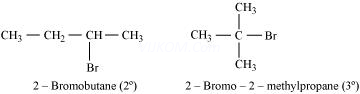
2-Bromobutane is ![]() alkylhalide whereas 2-bromo-2-methylpropane is
alkylhalide whereas 2-bromo-2-methylpropane is ![]() alkyl halide. Therefore, greater numbers of substituents are present in
alkyl halide. Therefore, greater numbers of substituents are present in ![]() alkyl halide than in
alkyl halide than in ![]() alkyl halide to hinder the approaching nucleophile. Hence, 2-bromobutane reacts more rapidly than 2-bromo-2-methylpropane by an
alkyl halide to hinder the approaching nucleophile. Hence, 2-bromobutane reacts more rapidly than 2-bromo-2-methylpropane by an ![]() mechanism.
mechanism.
(iii)
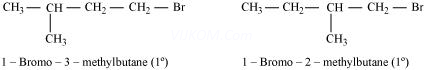
Both the alkyl halides are primary. However, the substituent ![]() is at a greater distance to the carbon atom linked to Br in 1-bromo-3-methylbutane than in 1-bromo-2-methylbutane. Therefore, the approaching nucleophile is less hindered in case of the former than in case of the latter. Hence, the former reacts faster than the latter by
is at a greater distance to the carbon atom linked to Br in 1-bromo-3-methylbutane than in 1-bromo-2-methylbutane. Therefore, the approaching nucleophile is less hindered in case of the former than in case of the latter. Hence, the former reacts faster than the latter by ![]() mechanism.
mechanism.
Ans 7. (i)

SN1 reaction proceeds via the formation of carbocation. The alkyl halide (I) is ![]() while (II) is
while (II) is![]() . Therefore, (I) forms
. Therefore, (I) forms ![]() carbocation while (II) forms
carbocation while (II) forms ![]() carbocation. Greater the stability of the carbocation, faster is the rate of SN1 reaction. Since
carbocation. Greater the stability of the carbocation, faster is the rate of SN1 reaction. Since ![]() carbocation is more stable than
carbocation is more stable than ![]() carbocation. (I), i.e. 2-chloro-2-methylpropane, undergoes faster SN1 reaction than (II) i.e., 3-chloropentane.
carbocation. (I), i.e. 2-chloro-2-methylpropane, undergoes faster SN1 reaction than (II) i.e., 3-chloropentane.
(ii)

The alkyl halide (I) is ![]() while (II) is
while (II) is![]() .
. ![]() carbocation is more stable than
carbocation is more stable than ![]() carbocation. Therefore, (I), 2-chloroheptane, undergoes faster
carbocation. Therefore, (I), 2-chloroheptane, undergoes faster ![]() reaction than (II), 1-chlorohexane.
reaction than (II), 1-chlorohexane.
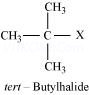
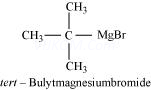
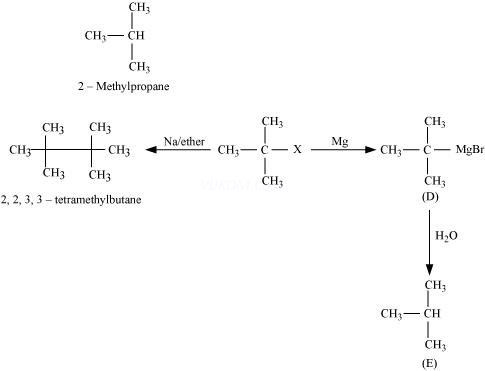
Ans 8.
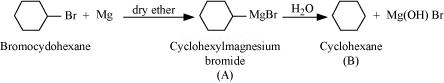
Since D of ![]() gets attached to the carbon atom to which MgBr is attached, C is
gets attached to the carbon atom to which MgBr is attached, C is
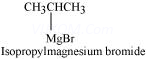
Therefore, the compound R – Br is
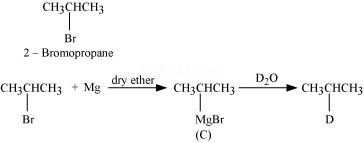
When an alkyl halide is treated with Na in the presence of ether, a hydrocarbon containing double the number of carbon atoms as present in the original halide is obtained as product. This is known as Wurtz reaction. Therefore, the halide, ![]() , is
, is
Therefore, compound D is
And, compound E is
Assertion and Reason Answers-
1. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
Explanation:
As the size of the alkyl groups increases, the SN2 reactivity decreases, further C – Cl bond is stronger and more difficult to cleave than C – Br bond. So CH3Br is more reactive than (CH3)2CHCl.
2. (c) Assertion is correct statement but reason is wrong statement.
Explanation:
Alkyl iodides in general turn brown due to liberation of I2 on decomposition by the action of air and light.
Case Study Answers-
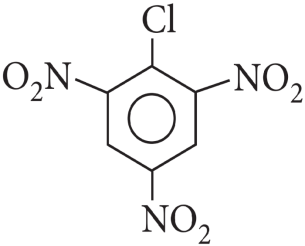
1. Answer :
Explanation:
When in aryl halides the electron withdrawing groups are attached at ortho and para positions to the chlorine atom then the removal of chlorine atom as Cll– ion becomes easy, therefore, 2,4,6-trinitro chlorobenzene is the most reactive among given aryl halides.
Explanation:
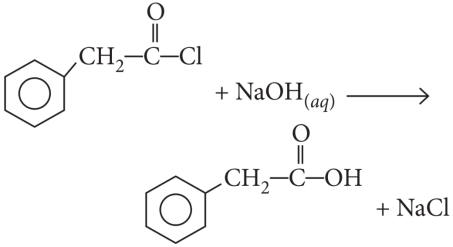
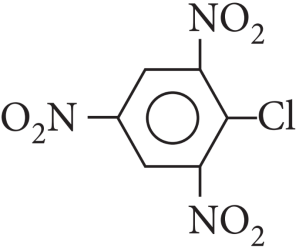
Explanation:
Cl in 2,4,6-trinitrochlorobenzene is activated by three NO2 groups at o, and p-positions and hence undergoes hydrolysis most readily.
Explanation:
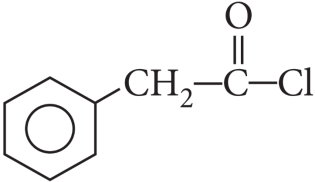
The order of reactivity follows the sequence: benzyl halides > alkyl halides> aryl halides. Out of chlorides and bromides, bromides are more reactive. Therefore, the correct order of reactivity is PhCH2Br (ii) > MeBr (i) > MeCl (iii) > p – MeOC6H4Br (iv).
2. Answer :