COORDINATION COMPOUNDS
COORDINATION COMPOUNDS
Coordination compounds are a special class of compounds in which the central metal atom is surrounded by ions or molecules beyond their normal valency. These are also referred to as complex compounds. These compounds are called Coordination compounds.
Many biologically important compounds are coordination compounds in which complicated organic species are bound to metal ions. The common examples are : haemoglobin which is a coordination compound of iron, chlorophyll which is a coordination compound of magnesium, vitamin B12 which is a coordination compound of cobalt etc.
The coordination compounds contain a central metal atom or ion surrounded by a number of oppositely charged ions or neutral molecules more than its normal valency.
For example: When aqueous ammonia is added to green solution of nickel chloride, NiCl2, the colour changes to purple. The Ni2+ ions almost disappear from the solution. The solution on evaporation, yields purple crystals corresponding to the formula [Ni(NH3)6]Cl2. Such a compound is called coordination (or complex) compound. The properties of the complex compound are completely different from those of Ni2+ ions or ammonia molecules.

When the compound is dissolved in water, it ionises to give a new species (Ni(NH3)6]2+. Such an ion is called complex ion.
[Ni(NH3)6]Cl2 → [Ni(NH3)6]2+ + 2Cl–
At this stage, it may be noted that the species in the square brackets does not ionise. It remains as single entity. It is known as complex entity.
Double Salts
Double salts are addition or molecular compounds which are formed by two apparently saturated compounds but they lose their identity when dissolved in water.
The common double salts are:
Mohr’s salt : FeSO4. (NH4)2SO4.6H2O
Potash alum : K2SO4.Al2(SO4)3.24H2O
Carnallite : KCl.MgCl2.6H2O
For example: Mohr’s salt dissolves in water and gives the characteristic properties of Fe2+, NH4+, and SO42- ions. Thus, double salts are stable in solid state but break up into constituents when dissolved in water.
FeSO4.(NH4)2SO4 → Fe2+ + (aq) + 2NH4+ (aq) + 2SO42-(aq)
On the other hand, the coordination compounds retain their identities in the solid state as well as when dissolved in water or any other solvent. Their properties are completely different from the constituents (metal and ions or molecules).
For example, [Ni(NH3)6]Cl2 does not show the properties of NiCl2 or ammonia. Similarly, complex ion such as [Fe(CN)6]4- of K4[Fe(CN)6] does not dissociate into Fe2+ and CN¯ ions.
Werner’s Coordination Theory
Alfred Werner a Swiss chemist, in 1892 prepared a large number of coordination compounds and studied their physical, chemical and isomeric behaviour by simple experimental techniques. He isolated cobalt compounds from the reaction of cobalt chloride and ammonia.
The earlier studies of cobalt complexes were precipitation reactions, conductance measurements and isomeric behaviour.
(1) Precipitation Studies
The number of ions furnished by a complex in a solution can be determined by precipitation reactions.
For example:
(a) The number of Cl‾ ions in a solution of various amines were determined by the treatment with silver nitrate solution. From the amount of white precipitate of AgCl formed per mole of the compound, the number of Cl‾ ions can be calculated.
(b) When the compound CoCl3.6NH3 is treated with excess of AgNO3 , 3 mol of AgCl are obtained from 1 mol of the compound i.e. all the three Cl¯ ions are precipitated.
(c) When the compound CoCl3.5NH3 is treated with excess of AgNO3, 2 mol of AgCl are obtained i.e., only two Cl¯ ions are precipitated. This means that the compound CoCl3.5NH3 has three ionizable chloride ions whereas in the compound CoCl3.5NH3 only two chlorine atoms are ionizable as Cl¯ ions.
CoCl3.6NH3 → 3 AgCl ( corresponding to 3 Cl¯ ions)
CoCl3.5NH3 → 2 AgCl ( corresponding to 2 Cl¯ ions)
Similarly, the number of chloride ions precipitated in the case of the compounds CoCl3.4NH3 and CoCl3.3NH3 have been found to be 1 and none.
(2) Conductance measurements
The measurement of molar conductances (^m) of solutions of coordination compounds helps to estimate the number of ions furnished by the compound in solution.
By comparing the molar conductance of the compounds with those of some known electrolytes, Werner was able to predict the number of ions present in the solution.
For example: The complex CoCl3.6NH3 behaved as 1:3 electrolyte, CoCl3.5NH3 as 1:2 electrolyte, CoCl3.4NH3 as 1:1 electrolyte.
(3) Isomers of compounds
Werner attempted to assign structures of different coordination compounds by comparison of the number of known isomers and the number of theoretically possible structures.
Postulates of Werner’s Coordination Theory
(1) In co-ordination compounds, metal atoms exhibit two types of valencies namely, the primary valency and the secondary valency. The primary valency is ionizable whereas the secondary valency is non ionizable. The primary valency corresponds to oxidation state and the secondary valency corresponds to coordination number.
(2) Every metal atom has a fixed number of secondary valencies i.e., it he fixed coordination number.
(3) The metal atom tends to satisfy both its primary as well as secondary valencies. Primary valencies are satisfied by negative ions whereas secondary valencies are satisfied either by negative ions or by neutral molecules. In certain cases, a negative ion may satisfy both types of valencies.
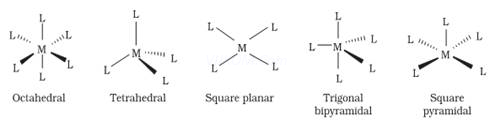
(4) The secondary valencies are always directed towards the fixed position in space and this leads to definite geometry of the coordination compound. Secondary valencies have characteristic spatial arrangements corresponding to different coordination numbers. Spatial arrangements are called coordination polyhedra.
For example: If a metal ion has six secondary valencies, these are arranged octahedrally around the central metal ion. If the metal ion has four secondary valencies, these are arranged in either tetrahedral or square planar arrangement around the central metal ion. The secondary valencies, thus, determine the stereochemistry of the complex.
Thus, a metal atom exhibits primary valencies in the formation of its salts (e.g., CoCl3 , AgNO3) while the metal atom exhibits its secondary valencies in the formation of its complex ions
e.g.: [Co(NH3)6]3+ , [Ag(NH3)2]+
Structures of Coordination Compounds on the Basis of Werner’s Theory
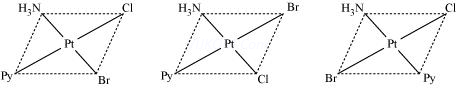
(1) CoCl3.6NH3 : Cobalt has primary valency (oxidation state) of three and secondary valency (coordination number) six. Secondary valencies are represented by thick lines (—) and primary valencies are shown by dotted lines (….). In the complex, all the 6 secondary valencies are occupied by six NH3 molecules. The Cl¯ ions are bonded to Co by three primary valencies. These chloride ions are ionisable and therefore can be precipitated on the addition of silver nitrate. The central metal ion and the neutral molecules or ions (ligands) satisfying secondary valencies are written in a square bracket while writing the formula of the complex compound.
Thus, the coordination compound may be formulated as [Co(NH3)6]Cl3 .
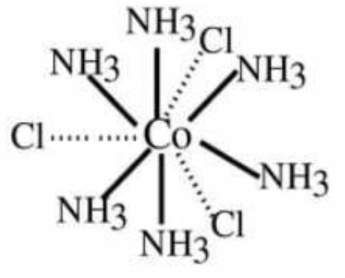
The primary valencies are ionizable and therefore, all the chloride ions would get precipitated on the addition of silver nitrate.
The species within the square brackets are also called coordination entities (or complexes). The ions outside the square brackets are called counter ions. Thus, in the coordination compound [Co(NH3)6Cl]3, [Co(NH3)6]3+ represents coordination entity and 3Cl¯ ions represent counter ions
The ionisation of the coordination compound is written as:
[Co(NH3)6Cl]3⇔ [Co(NH3)6]3+ + 3Cl¯
(2) CoCl3.5NH3 : In this compound, the coordination number of cobalt is 6 but now five positions are occupied by NH3 molecules and the sixth position by one of the chloride ions.This chloride ion has dual character as it satisfies secondary as well as a primary valency as indicated by a full line as well as a dotted line.The two Cl¯ ions satisfy the remaining two primary valencies of cobalt. This satisfies 6 secondary and 3 primary valencies of cobalt.However, on ionisation, only two Cl¯ ions will be precipitated because one Cl- ion which also satisfied secondary valency, will not be precipitated.
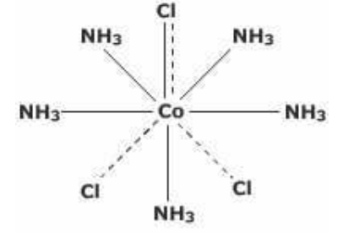
Thus, the coordination compound may be formulated as [CoCl(NH3)5]Cl2 which has [CoCl(NH3)5]2+ complex entity and 2Cl‾ ions as counter ions. The ionisation of the coordination compound may be written as:
[CoCl(NH3)5]Cl2⇔ [CoCl(NH3)5]2+ + 2Cl‾
(3) CoCl3.4NH3 : In the compound CoCl3.4NH3 , two chloride ions exhibit dual character of satisfying both primary and secondary valencies.It will give precipitate with silver nitrate corresponding to only one Cl‾ ion and the number of ions in this case is 2.
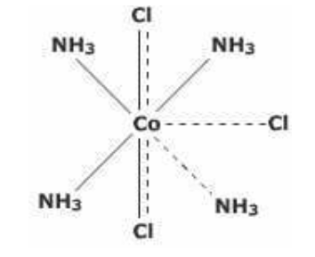
It may be formulated as
[CoCl2(NH3)4]Cl⇔ [CoCl2(NH3)4]+ + Cl‾
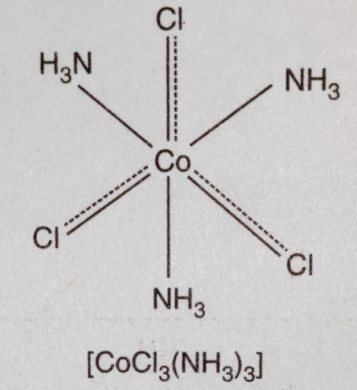
(4) CoCl3.NH3 : In the compound CoCl3.3NH3 ,three chloride ions satisfy primary and secondary valencies. All the chloride ions are non-ionisable and will not be precipitated by the addition of AgNO3. Therefore, the coordination compound behaves as neutral non- conducting molecule.
It may be formulated as [CoCl3(NH3)3] and does not ionise.
[CoCl3(NH3)3]⇔ Does not ionize
Differencesbetweencoordinationcompoundanddoublebond
|
Coordinationcompound |
Doublesalt |
|
A coordination compound contains acentral metal atom or ion surroundedby several oppositely charged ions orneutralmolecules. Theseions or molecules re-bonded to the metalatom orionbyacoordinatebond. |
Whentwosaltsinstoichiometricratioare crystallised together from theirsaturated solution, they are calleddoublesalts. |
|
Example:K4[Fe(CN)6] |
Example:FeSO4.(NH4)2SO4.6H2O (Mohr’ssalt) |
|
They do not dissociate into simpleionswhendissolvedinwater. |
Theydissociateintosimpleionswhendissolvedinwater. |
nitrogenatoms, and oxalate ion has two oxygen atoms which can bind with the metal atom.
|
Primaryvalence |
Secondaryvalence |
|
Thisvalenceisnormallyionisable. |
Thisvalenceisnon-ionisable. |
|
Itisequaltothepositivechargeon the centralmetalatom. |
Thesecondaryvalencyequalstothenumber of ligand atoms coordinated tothemetal.Itisalsocalledthecoordinationnumber ofthemetal. |
|
Thesevalenciesaresatisfiedbynegativelychargedions. |
It is commonly satisfied by neutral andnegativelycharged,sometimesbypositivelychargedligands. |
|
Example:InCrCl3,theprimaryvalencyisthree.Itisequaltothe oxidationstateofthecentralmetalion. |
|
Examples: [Co(NH3)5Br]SO4and[Co(NH3)5SO4] Br
Examples:[Co(NH3)5ONO]Cl2and[Co(NH3)5NO2]Cl2
Isomerism in Coordination Compounds
Isomerism
Two or more compounds having the same molecular formula but different arrangement of atoms are called isomers and the phenomenon is called isomerism. Because of different arrangement of atoms, isomers differ in one or more physical or chemical properties.
Isomers can be broadly classified into two major categories :
(A) Structural isomers
(B) Stereoisomers
Structural isomers
1. Ionisation isomerism
2. Hydrate isomerism
3. Coordination isomerism
4. Linkage isomerism
Stereoisomers
1. Geometrical isomerism
2. Optical isomerism
(A) Structural isomerism
The isomers which have same molecular formula but different structural arrangement of atoms or groups of atoms around the central metal ion are called structural isomers.
(1) Ionisation isomerism
The compounds which have same molecular formula but give different ions in solution are called ionisation isomers. In this type of isomerism, the difference arises from the interchange of groups within or outside the coordination entity. This type of isomerism occurs when the counter ion in a coordination compound is itself a potential ligand.
For example: There are two isomers of the compound of the formula Co(NH3)5BrSO4
(a) One of these is red-violet and forms a precipitate with BaCl2 indicating that sulphate ion is outside the coordination entity.
(b) The second one is red and does not form precipitate with BaCl2 but forms a precipitate of AgBr with silver nitrate indicating that bromide ion is outside the coordination entity.
The structures of the two compounds and their mode of ionisation are :
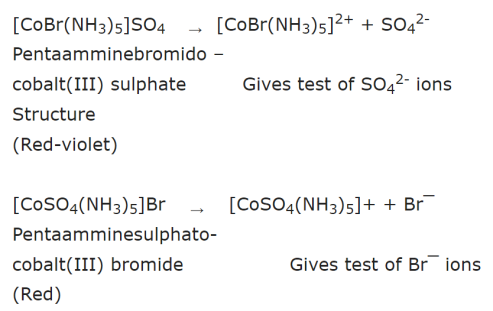
Other compounds showing this type of isomerism are:
(i) [CoCl2(NH3)4]NO2 and [CoCl(NO2)(NH3)4]Cl
(ii) [Co(NO3)(NH3)]SO4 and [Co(SO4)(NH3)5]NO3
(iii) [PtCl2(NH3)4]Br2 and [PtBr2(NH3)4]Cl2
(iv) [CoCl(NO2)(NH3)4]Cl and [CoCl2(NH3)4]NO2
(2) Solvate or Hydrate isomerism
The compounds which have the same molecular formula but differ by whether or not a solvent molecule is directly bonded to the metal ion or merely present as free solvent molecules in the crystal lattice are called solvate isomers.
It is also known as hydrate isomerism where water is involved as a solvent.Thus, hydrate isomers differ in the number of water molecules present as ligands or as molecules of hydration.
In type of isomerism water molecules may occur inside and outside the coordination sphere as a coordinated group or a water of hydration.
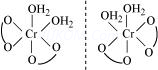
For example, there are three isomers having the molecular formula CrCl3⋅6H2O.
These are :
(CrCl3(H2O)3], [CrCl(H2O)5]Cl2⋅H2O and [CrCl2(H2O)4]Cl⋅2H2O
(i) [Cr(H2O)6]Cl3 : It does not lose water when treated with conc. H2SO4 and three chloride ions are precipitated with AgNO3.
(ii) [CrCl(H2O)5]Cl2⋅H2O
Blue green
It loses one water molecule when treated with conc. H2SO4 and 2Cl¯ ions are precipitated with AgNO3
(iii) (CrCl2(H2O)4]Cl.2H2O : It loses two water molecules on treatment with conc. H2SO4 dark green and one Cl¯ ion is precipitated with AgNO3.
Similarly, the following two isomers are hydrate isomers :
[CoCl(en)2(H2O)]Cl2 and [CoCl2(en)2]Cl.H2O
[CoCl(H2O)(NH3)4]Cl2 and [CoCl2(NH3)4]Cl.H2O
[CrCl2(C2H5N)2(H2O)2]Cl and [CrCl3(C2H5N5)2H2O].H2O
(3) Coordination isomerism
The type of isomerism occurs in compounds containing both cationic and anionic entities and the isomers differ in the distribution of ligands in the coordination entity of cationic and anionic parts. This type of isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in the complex.
The examples are:
(i) [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6] [Co(CN)6]
(ii) [Cu(NH3)4[PtCl4] and[Pt(NH3)4][CuCl4]
This type of isomerism is also shown by compounds in which the metal ion is the same in both cationic and anionic complexes.
For example :
(i) [Cr(NH3)6][Cr(CN)6] and [Cr(CN)2(NH3)4][Cr(CN)4(NH3)2]
(ii) [(Pt(NH3)4] [PtCl4] and [PtCl(NH3)3][PtCl3(NH3)]
(4) Linkage isomerism
The compounds which have the same molecular formula but differ in the mode of attachment of a ligand to the metal atom or ion are called linkage isomers.
For example: In NO2¯ ion, the nitrogen atom as well as the oxygen atom can donate their lone pairs. This gives rise to isomerism.
If nitrogen donates its lone pair, one particular compound will be formed.
If oxygen donates its lone pair, a different compound (although having the same molecular formula) is obtained. If the bonding is through N, the ligand is named as nitrito-N(or nitro) and if it is through O, it is named as nitrito-O (or nitrito).
NO2¯ nitrito-N (or nitro)
ONO¯ nitrito-O (or nitrito)
For example: Jorgensen discovered such behaviour in the complex [(Co(NH3)5(NO2)]Cl. He prepared two different pentaamminecobalt(II) chloride each containing the NO2 group in the complex ion. These are:
The unidentate ligands which can bind to the central atom through two donor atoms are also called ambidentate ligands.
Other examples of ligands are:
CN Cyano (through C)
NC Isocyano (through N)
SCN Thiocyanato (through S)
NCS Isothiocyanato (through N)
(B) Stereoisomers
Stereoisomers are those isomers which have the same position of atoms or groups but they differ in the spatial arrangements around the central atom. Two types of isomerism viz., geometrical isomerism and optical isomerism.
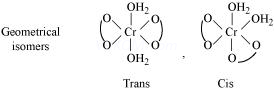
(1) Geometrical isomerism
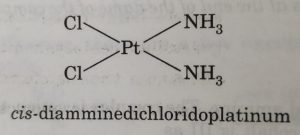
Geometrical isomerism arises in heteroleptic complexes due to ligands occupying different positions around the central ion. The ligands occupy positions either adjacent to one another or opposite to one another. These are referred to as cis- form (ligands occupy adjacent positions) and trans- form (ligands occupy opposite positions). This type of isomerism is, therefore, also referred to as cis-trans isomerism.
(a) Geometrical isomerism in complexes of coordination number 4
The complexes having coordination number 4 adopt tetrahedral or square planar geometry. The geometrical isomerism is not possible in tetrahedral complexes. This is because in tetrahedral geometry all the positions adjacent to one another in these complexes.
However, square planar complexes show geometrical isomerism.
Example:
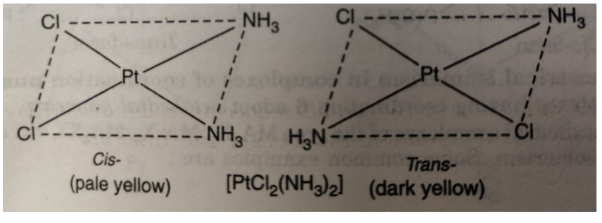
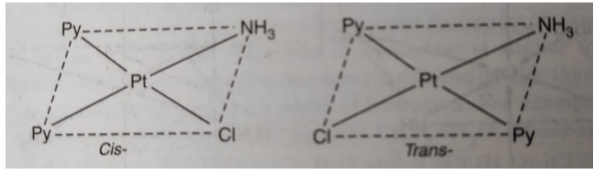
(ii) [PtCl(C5H5N)2 (NH3)] exists in cis and trans form as:
(iii) Square planar complexes of the type MABCD show three isomers. The structures of these isomers can be written by fixing the position of one ligand (say A) and placing the other ligands B, C and D trans to it.
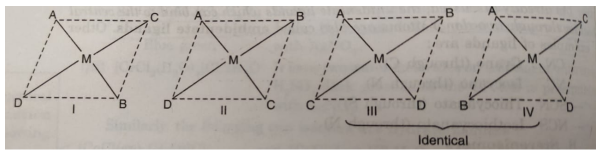
The complex [Pt(NO2)(py) (NH2OH)(NH3)]+ exists in three geometrical isomers as represented below:
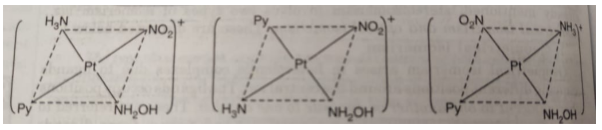
(iv) Geometrical isomerism cannot occur in complexes of the type MA4 , MA3B or MAB3 because all possible spatial arrangements for any of these complexes will be exactly equivalent.
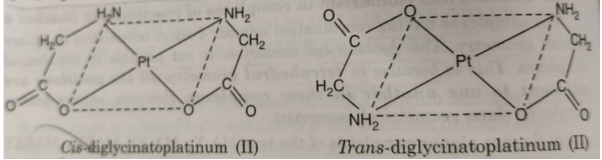
(v) The square planar complexes containing unsymmetrical bidentate ligands such as [M(AB)2] also show geometrical isomerism. For example, the complex [Pt(gly)2] where gly = NH2CH2COO¯ exists in cis and trans form :
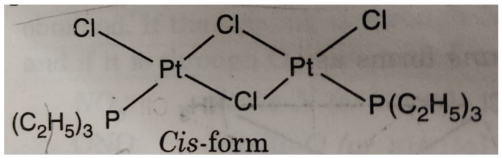
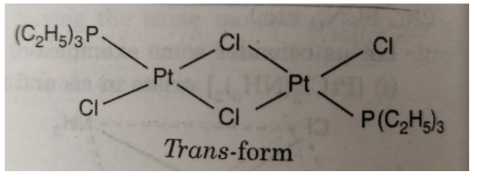
(vi) Geometrical isomerism is also shown by bridged binuclear complexes of the type M2A2X4. For example: the complex [PtCl2 P(C2H5)3]2 exhibits geometrical isomers as :
IUPAC Nomenclature of Coordination Compounds
Rules for Writing Formula
The formula of a compound is a shorthand method used to provide basic information about the constitution of a compound in a concise and convenient manner.
(1) The formula of the cation whether simple or complex is written first followed by that of the anion.
(2) The coordination entity is written in square brackets.
(3) The sequence of symbols within the coordination entity is : first the symbol of the central metal atom followed by ligands in alphabetical order.
The ligands in coordination entity are arranged as
(a) The different ligands are arranged alphabetically according to the first symbol of their formulae. For example, H2O , NH3, NO3¯, SO42- and OH¯ etc. are cited at H, N, N, O, S and O.
(b) When the two ligands have same defining atom, the ligand with fewer such atoms is cited first followed by the ligand having more atoms. For example, NH3 precedes N2
(c) If the numbers of defining atoms are equal, subsequent symbol decides the sequence.
For example NH2¯ precedes NO2¯ because H comes before O.
(d) Polydentate ligands are also listed alphabetically. In case of abbreviated ligand, the first letter of the abbreviation is used to determine the position of the ligand in alphabetical order.
(e) The formula for the co-ordination entity, whether charged or not, is enclosed in square brackets. Polyatomic ligands are enclosed in parentheses (), but all ligands are written without any separation in between.
(f) There should be no space between the representations of ionic species within the formula.
(g) Sometimes abbreviations are used for formulae of the ligands. These abbreviations should be in lower case and enclosed in parenthesis.
For example, py is used for pyridine and en is used for ethane-1, 2-diamine or ethylene diamine.
(h) The number of cations or anions to be written in the formula is calculated on the basis that total positive charge must be equal to total negative charge.
(i) When the formula of the charged coordination entity is written without the formula of the counter ion, the charge is indicated outside the square brackets as a right superscript with the number before the sign (+ or -).
For example,
[Ni(NH3)6]2+, [Co(CN)6]3- , [CoCl(NH3)5]2+, etc.
Some common examples are :
K3[Fe(CN)6]
[CoCl2(NH3)4]Cl Cl is cited first than NH3
[Co(H2O)2(NH3)4]Cl2 H is cited before N
[Pt Cl2(C5H5N)NH3] Among neutral ligands C5H5N and NH3, C5H5N precedes NH3
Rules for naming the coordination compounds
(1) Order of naming ions
In ionic complexes, the cation is named first and then the anion.
NaCl : sodium chloride
Non-ionic complexes are given a one word name.
(2) Naming the coordination entity
In naming the coordination entity, the ligands are named first and then the central metal ion.
(3) Names of ligands
The names of anionic ligands (organic or inorganic) end in o-. In general, if the anionic ligand name ends in -ide, -ite or -ate, the final ‘e’ is replaced by ’o’ giving-ido,-ito and-ato respectively. For inorganic anionic ligands containing numerical prefixes such as triphosphate enclosing marks () are added. The names of positive ligands end in -ium. The neutral ligands are named as such.
For example :
(i) Negative ligands end in -o :
F- fluorido
Cl- chlorido
Br¯ bromido
NO2¯ nitrito-N
ONO¯ nitrito-O
SO42¯ sulphato
OH- hydroxo
C2O42‾ oxalato
CN– cyano
CH3COO¯ acetato
SCN- thiocyanato
O22– peroxo
O2- oxo
N3– nitrido
P3- phosphido
N3¯ azido
NCS¯ isothiocyanato
H¯ hydrido
CO32– carbonato
NO3¯ nitrato
NH2¯ amido
NH2- imido
ClO3¯ Chlorato
S2O32– thiosulphato
(ii) positive ligands end in -ium
NO+nitrosonium
NH2NH3+ hydrazinium
NO2+ nitronium
(iii) neutral ligands are named as such
NH2CH2CH2NH2 ethane-1,2-diamine or ethylenediamine
C6H5N pyridine (py)
(C6H5)3P triphenylphosphine
PH3 phosphine
CS thiocarbonyl
H2NCSNH2 thiourea (tu)
dipyridyl (dipy)
However, there are a few exceptions in naming neutral ligands. For example,
H2O aqua
NH3 ammine
NO nitrosyl
CO carbonyl
(4) Order of naming ligands
When more than one type of ligands are present, they are named in alphabetical order of preference without any consideration of charge.
For example : In the complex [CoCl(NO2)(NH3)4]+, the ligands are named in the order :
ammine, chlorido and nitrito-N. Similarly, in the complex K3[Fe(CN)5NO], the ligands are named as cyano and nitrosyl.
(5) Numerical prefixes to indicate number of ligands
When more than one ligands of a particular kind are present in the complex, the di-, tri-, tetra-, penta-, hexa-, etc. are used to indicate their number: two, three, four, five and six respectively.
When the name of the ligand, includes the numerical prefix (di, tri, tetra), the prefixes bis, tris, tetrakis are used for two, three, four ligands, respectively. Such ligands are called complexligands.
For example: to indicate two simple ligands such as chloro, bromo, ammine, oxalato, etc., we use the prefix di but to indicate two complex ligands such as ethylenediamine we use the prefix bis (ethylenediamine) or bis (1,2-ethanediamine).
The name of the complex ligand is given in brackets.
For example :
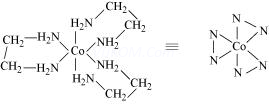
[Co(en)3]3+ tris(ethane-1,2,-diamine)cobalt(III) ion
[NiCl2(PPh3)2] dichloridobis (triphenylphosphine)nickel(II)
(6) Ending of names
When the complex is anionic, the name of the central metal atom ends in -ate. For cationic and neutral complexes, the name of the metal is written without any characteristic ending.
For example, the cationic complex [Co(NH3)6]Cl3 is named without characteristic ending of the name of the metal as :
[Co(NH3)6]Cl3 hexaamminecobalt (III) chloride
The coordination compound, K[PtCl5(NH3)] which contains the anionic complex [PtCl5(NH3)]¯ is named with ending of the name of the metal as -ate.
K[PtCl5(NH3)] potassium amminepentachloridoplatinate (IV)
Similarly, the anionic complex Ca2[Fe(CN)6] is named as calcium hexacyanoferrate (II).
[Co(SCN)4]2– tetrathiocyanatocobaltate(II) ion.
For anionic complexes the Latin names of certain metals are commonly used.
For example: ferrate for Fe, cuperate for Cu, argentate for Ag, aurate for Au, stannate for
Sn, etc.
However, if the complex is cationic or neutral the name of the metal is given as such e.g., iron for Fe, silver for Ag, gold for Au, copper for Cu, etc.
For example:
K3[Fe(CN)6] Potassium hexacyanoferrate (III)
[Fe(CO)5] Pentacarbonyliron (0)
(7) Oxidation state of central metal ion
The oxidation state of the central metal ion is designated by a Roman numeral (such as II, III, IV) in the brackets at the end of the name of the complex without a gap between the two. Let us discuss some examples:
(i) CoCl(NH3)5]Cl2
In this case the ligands are chloro and ammine. The complex is cation and chloride is anion. The oxidation state of cobalt is III as
x+ 5(0) – 1- 2 = 0
or
x = +3.
Its name is pentaamminechloridocobalt(III) chloride
(ii) K3[Fe(CN)6]
In this case, the ligands are cyano. The complex is anionic. The oxidation state of iron is +3 as 3 (+1) +x + 6(-1) = 0
The name of the complex is potassium hexacyanoferrate(III). If the complex containing the central ion, Fe3+ is anionic, the Latin name of metal is used i.e. ferrate.
(iii) [Co(H2NCH2CH2NH2)3] (SO4)3
In this case, ligands are ethane-1, 2-diamine (or ethylenediamine). The complex is cationic. The oxidation state of cobalt is +3 as
[x + 3 x 0]2 + 3 (-2) = 0 or
2x = +6 or
x = +3
The name of the complex is tris (ethane-1, 2-diamine) cobalt(II) sulphate.
tris is used because the ligand is complex ligand.
(iv) [Ag(NH3)2] [Ag(CN)2]
Both cation and anion are complexes. The oxidation state of silver in both cationic and anionic complexes is +1. The name of the complex is diamminesilver (I) dicyanoargentate (I)
(8) Point of attachment
When a ligand can coordinate through more than one atom, then the point of attachment of the ligand is indicated by putting the symbol of the atom through which coordination occurs after the name of the ligand. For example: NO2¯ can coordinate through -N or -O. If it coordinates through N, it is called nitrito -N (or simply as nitro). If it coordinates through O, -ONO, it is called nitrito -O or simply as nitrito.
NO2¯ (through N)
-ONO¯ (through O)
Nitrito – N
Nitrito – O
SCN¯ (through S) thiocyanato
NCS (through N) isothiocyanato
For example,
[Co(NO2)3(NH3)3] triamminetrinitrito-N-cobalt(III)
[Co(ONO)(NH3)5]SO4 pentaamminenitrito-O-cobalt(III) sulphate
(9) Naming geometrical isomers
Geometrical isomers are named by the use of the terms cis-to designate adjacent positions and trans- to designate opposite positions.
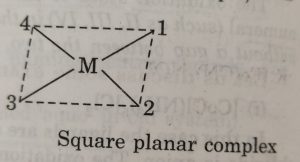
For example: in square planar complexes shown below
(a) the positions 1, 4 ; 1, 2 ; 2, 3 and 3, 4 are cis-
(b) 1,3 and 2, 4 are trans
In naming these complexes, cis or trans is written before the names of these compounds. Similarly, for octahedral complexes, cis- and trans are used in isomerism in coordination compounds.
(10) Naming optical isomers
Optically active compounds are designated by the symbols (+) or d -for dextrorotatory and (-) or l- for laevorotatory.
For example : d- K3[Cr(C2O4)3] Potassium (+) trioxalatochromate (III)
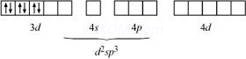
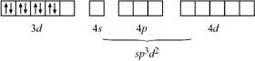
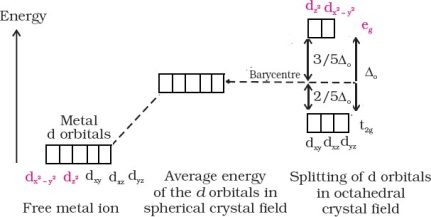
According to this theory, the metal atom or ion under the influence ofligandscanuseits(n −1)d, ns, nporns,nporndorbitalsforhybridization to yield a set of equivalent orbitals of definite geometry such as octahedral, tetrahedral and square planar.These hybridised orbitals are allowed to overlap with ligand orbitals which can donate electron pairs forbonding.
|
Coordination number |
Typeof hybridisation |
Distributionofhybridorbitalsinspace |
|
4 |
sp3 |
Tetrahedral |
|
4 |
dsp2 |
Squareplanar |
|
5 |
sp3d |
Trigonalbipyramidal |
|
6 |
sp3d2(nd orbitals areinvolved; outer orbitalcomplex or high-spin or spin-freecomplex) |
Octahedral |
|
7 |
d2sp3 [(n − 1)d orbitals areinvolved; inner orbitalcomplexorlow-spinorspin-pairedcomplex] |
Octahedral |
Magnetic momentμ=√n(n+2)where n is the number of unpaired electrons.
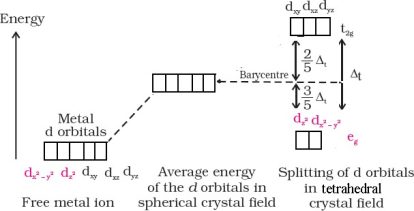 Crystalfieldsplittingintetrahedralcoordinationcomplexes
Crystalfieldsplittingintetrahedralcoordinationcomplexes
Δt=49Δ0
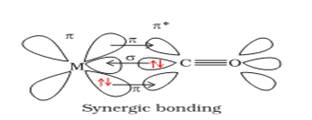
Themetal–carbonbondinmetalcarbonyls possessesbothσandπcharacters.Themetal–carbonbondin metal carbonyls possess both s and p characters. The M–C σ bond is formed by the donation of alonepairofelectronsfromthecarbonylcarbonintoavacant orbitalofthemetal.The M–Cπbondisformedbythedonationofapairof electronsfrom afilleddorbitalof metalintothevacantanti-bondingπ*orbitalofcarbonmonoxide. ThemetaltoligandbondingcreatesasynergiceffectwhichstrengthensthebondbetweenCOandthemetal.
Important Questions
Multiple Choice questions-
1. IUPAC name of [Pt(NH3)3 Br (NO2) Cl] Cl isw
(a) triamminechlorodibromidoplatinum (IV) chloride
(b) triamminechloridobromidonitrochloride- platinum (IV) chloride
(c) triamminebromidochloridonitroplatinum (IV) chloride
(d) triamminenitrochlorobromoplatinum (IV) chloride
2. Trunbull’s blue is
(a) Ferricyanide
(b) Ferrous ferricyanide
(c) Ferrous cyanide
(d) Fe3[Fe(CN)6]4
3. Primary and secondary valency of Pt in [Pt(en)2Cl2] are
(a) 4, 4
(b) 4, 6
(c) 6, 4
(d) 2, 6
4. The complex ions [Co(NH3)5(NO2)]2+ and [Co(NH3)5 (ONO)]2+ are called
(a) Ionization isomers
(b) Linkage isomers
(c) Co-ordination isomers
(d) Geometrical isomers
5. Which of the following has square planar structure?
(a) [NiCl4]2-
(b) [Ni(CO)4]
(c) [Ni(CN)4]2-
(d) None of these
6. Which of the following has magnesium?
(a) Chlorophll
(b) Haemocyanin
(c) Carbonic anhydrate
(d) Vitamin B12
7. Mohr’s salt is
(a) Fe2(SO4) 3 . (NH4)2SO4 . 6H2O
(b) FeSO4 . (NH4)2 . SO4 . 6H2O
(c) MgSO4 . 7H2O
(d) FeSO4 . 7H2O
8. Which of the following shall form an octahedral complex?
(a) d4 (low spin)
(b) d8 (high spin)
(c) d6 (low spin)
(d) All of these
9. EDTA is used for the estimation of
(a) Na+ and K+ ions
(b) Cl– and Br– ions
(c) Cu2+ and Cs+ ions
(d) Ca2+ and Mg2+ ions
10. The solution of the complex [Cu(NH3)4] SO4 in water will
(a) give the tests of Cu2+ ion
(b) give the tests of NH3
(c) give the tests of SO42- ions
(d) not give the tests of any of the above
Very Short Questions-
1. What is the shape of![]() ?
?
2. What do you understand by stability of a complex and instability constant of coordination compounds?
3. How is EDTA used in estimation of hardness of water?
4. Explain the role of complexes in metallurgy with an example.
5. How is excess of copper and iron removed from body?
6. Define – isomerism.
7. Indicate the types of isomerisms shown by the complex![]() ?
?
8. Give an example of coordination isomerism?
9. What are complex compounds?
10. Give some examples of coordination compounds.
Short Questions-
1.Explain the synergic bonding in metal carbonyls.
2. Give some examples showing importance of complexes in biological system?
3.Give examples of complexes in
a) Chemical analysis
b) Industries
4.Distinguish between homoleptic and heteroleptic ligands.
5.What are the different shapes or coordination polyhedral in the complexes?
6.What is the difference between a double salt and a complex? Explain with an example.
7. Predict the number of unpaired electrons in the square planar ![]() ion.
ion.
8. Write all the geometrical isomers of ![]() and how many of these will exhibit optical isomers?
and how many of these will exhibit optical isomers?
9. What is spectrochemical series? Explain the difference between a weak field ligand and a strong field ligand.
10.A solution of ![]() is green but a solution of
is green but a solution of ![]() is colourless. Explain.
is colourless. Explain.
Long Questions-
1. What are ligands? Explain different types of ligands.
2. Write the formulas for the following coordination compounds:
(i) Tetraamminediaquacobalt (III) chloride
(ii) Potassium tetracyanonickelate (II)
(iii) Tris(ethane-1,2-diamine) chromium (III) chloride
(iv) Amminebromidochloridonitrito-N-platinate (II)
(v) Dichloridobis (ethane-1,2-diamine)platinum(IV) nitrate
(vi) Iron (III) hexacyanoferrate (II)
3. Write the IUPAC names of the following coordination compounds:
(i) ![]() (ii)
(ii) ![]()
(iii) ![]() (iv)
(iv) ![]()
(v) ![]() (vi)
(vi) ![]()
4. Indicate the types of isomerism exhibited by the following complexes and draw the structures for these isomers:
(i) ![]()
(ii) ![]()
(iii) ![]()
(iv) ![]()
5. Explain on the basis of valence bond theory that ![]() ion with square planar structure is diamagnetic and the
ion with square planar structure is diamagnetic and the ![]() ion with tetrahedral geometry is paramagnetic.
ion with tetrahedral geometry is paramagnetic.
6. ![]() is paramagnetic while
is paramagnetic while ![]() is diamagnetic though both are tetrahedral. Why?
is diamagnetic though both are tetrahedral. Why?
7. ![]() is strongly paramagnetic whereas
is strongly paramagnetic whereas ![]() is weakly paramagnetic. Explain.
is weakly paramagnetic. Explain.
8. Explain ![]() is an inner orbital complex whereas
is an inner orbital complex whereas ![]() is an outer orbital complex.
is an outer orbital complex.
Assertion and Reason Questions-
1. In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Zeise’s salt is a ππ-bonded organometallic compound.
Reason: The oxidation number of platinum in Zeise’s salt is +2.
2. In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: The second and third transition series elements have lesser tendency to fonn low spin complex as compared to the first transition series.
Reason: The CFSE (Δ0)is more for 5d and 4d.
Case Study Questions-
1. Read the passage given below and answer the following questions:
Metal carbonyl is an example of coordination compounds in which carbon monoxide (CO) acts as ligand. These are also called homoleptic carbonyls. These compounds contain both σ and π character. Some carbonyls have metal-metal bonds. The reactivity of metal carbonyls is due to (i) the metal centre and (ii) the CO ligands. CO is capable of accepting an appreciable amount of electron density from the metal atom into their empty π or π−orbital. These types of ligands are called π−accepter or π−acid ligands. These interactions increases the Δ0 value.
The following questions are multiple choice questions. Choose the most appropriate answer:
2. Read the passage given below and answer the following questions:
Coordination compounds are formulated and named according to the IUPAC system.
Few rules for naming coordination compounds are:
The following questions are multiple choice questions. Choose the most appropriate answer:
MCQ Answers-
Very Short Answers-
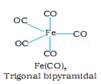
Ans 1.
![]()
Geometry = Trigonal bipyramidal/span>
Ans 2. The stability of a complex in solution is the degree of association between the two species involved in the state of equilibrium. The instability constant is reciprocal of the formation constant. It is also called dissociation constant.
Ans 3. Hard water is titrated with ![]() complex for estimation of its hardness. During the process, the
complex for estimation of its hardness. During the process, the ![]() ions form complex with EDTA replacing
ions form complex with EDTA replacing![]() . The method is based on the difference in the stability constant of calcium and magnesium complexes.
. The method is based on the difference in the stability constant of calcium and magnesium complexes.
Ans 4. Some extraction processes make use of complex formation e.g. during metallurgy of Gold, it combines with cyanide in the presence of oxygen and water to form the complex ![]() in aqueous solution by addition of zinc.
in aqueous solution by addition of zinc.
Ans 5. Excess of copper and iron are removed by chelating Ligands D – penicillamine and deferoxamine B through the formation of coordination compounds.
Ans 6. Isomerism is the phenomenon of existence of two or more compounds with same chemical formula but a different arrangement of atoms.
Ans 7. Both geometrical and optical isomerisms will be present.
Ans 8. Example of coordination isomerism is
![]() and
and ![]()
Ans 9. Complex compounds or coordination compounds are those compounds in which the metal atoms are bound to a number of anions or neutral molecules.
Ans 10. Examples of coordination compounds are chlorophyll, haemoglobin, and vitamin![]()
Short Answers-
Ans 1. The metal – carbon bond in metal carbonyls has both s & P- character. The M – C ![]() bond is formed by donation of lone pair of electrons of carbonyl carbon into a vacant orbital of metal. The M – C
bond is formed by donation of lone pair of electrons of carbonyl carbon into a vacant orbital of metal. The M – C ![]() bond is formed by the donation of a pair of electrons from a filled d- orbital of metal to the vacant
bond is formed by the donation of a pair of electrons from a filled d- orbital of metal to the vacant ![]() orbital of carbon monoxide. The metal to ligand bonding creates a synergic effect that strengthens the bond.
orbital of carbon monoxide. The metal to ligand bonding creates a synergic effect that strengthens the bond.
Ans 2. Examples of complexes in biological system-
1. Chlorophyll is a complex of Mg.
2. Haemoglobin is a complex of iron.
3. Cyanocobalamin, Vit![]() , is a complex of cobalt.
, is a complex of cobalt.
Ans 3. (a) Chemical analysis – Qualitative and Quantitative analysis methods involve use of Ligands like EDTA, DMG etc.
(b) Industries – Hydrogenation of alkenes is done by using a sodium complex called Wilkinson catalyst. In black and white photography, silver complexes are used.
Ans 4. Homoleptic complexes are those in which only one type of ligand or donor group is present e.g. ![]() has only
has only ![]() as ligand. Whereas heteroleptic complexes are those in which different types of ligands are present eg.
as ligand. Whereas heteroleptic complexes are those in which different types of ligands are present eg. ![]() has two type of ligands- NH3 and Cl–.
has two type of ligands- NH3 and Cl–.
Ans 5. The various coordination polyhedra are –
Ans 6. Double salts dissociate completely into simple ions when dissolved in water e.g., Mohr salt, ![]() ,
, ![]() will dissolve in water and give ferrous, ammonium and sulphate ions. On the other hand, the complex ions do not completely dissociate into all constituent ions e.g.
will dissolve in water and give ferrous, ammonium and sulphate ions. On the other hand, the complex ions do not completely dissociate into all constituent ions e.g.![]() ] will dissociate to give potassium ions and
] will dissociate to give potassium ions and ![]() ions only.
ions only.
Ans 7.![]()
In this complex, Pt is in the +2 state. It forms a square planar structure. This means that it undergoes ![]() hybridization. Now, the electronic configuration of Pd(+2) is
hybridization. Now, the electronic configuration of Pd(+2) is ![]() .
.
![]()
![]() being a strong field ligand causes the pairing of unpaired electrons. Hence, there are no unpaired electrons in
being a strong field ligand causes the pairing of unpaired electrons. Hence, there are no unpaired electrons in ![]() .
.
Ans 8.
![]()
From the above isomers, none will exhibit optical isomers. Tetrahedral complexes rarely show optical isomerization. They do so only in the presence of unsymmetrical chelating agents.
Ans 9. A spectrochemical series is the arrangement of common ligands in the increasing order of their crystal-field splitting energy (CFSE) values. The ligands present on the R.H.S of the series are strong field ligands while that on the L.H.S are weak field ligands. Also, strong field ligands cause higher splitting in the d orbitals than weak field ligands.
![]()
![]()
![]()
Ans 10. In ![]() ,
, ![]() is a weak field ligand. Therefore, there are unpaired electrons in
is a weak field ligand. Therefore, there are unpaired electrons in ![]() . In this complex, the d electrons from the lower energy level can be excited to the higher energy level i.e., the possibility of d – d transition is present. Hence,
. In this complex, the d electrons from the lower energy level can be excited to the higher energy level i.e., the possibility of d – d transition is present. Hence, ![]() is coloured.
is coloured.
In ![]() , the electrons are all paired as
, the electrons are all paired as ![]() is a strong field ligand. Therefore, d-d transition is not possible in
is a strong field ligand. Therefore, d-d transition is not possible in ![]() . Hence, it is colourless.
. Hence, it is colourless.
Long Answers-
Ans 1. The ions or molecules bound to central atom or ion in the coordination entity are ligands e.g ![]() has six
has six ![]() ligands.
ligands.
Types: –
(1) On the basis of charges on them ligands can be negative, positive (e.g.![]() ,
, ![]() etc.) or neutral (e.g. CO,
etc.) or neutral (e.g. CO, ![]() ,
,![]() ).
).
(2) On the basis of their donor atoms ligands can be monodentate or unidentate (one donor atom) e.g-![]() ,
, ![]() ,
, ![]() etc, or didentate ligands (two donor atoms)
etc, or didentate ligands (two donor atoms) ![]() or
or ![]() etc. or polydentate (several donor atoms) e.g
etc. or polydentate (several donor atoms) e.g ![]() is a hexadentate ligand.
is a hexadentate ligand.
(3) Ligands which can ligate through two different atoms are called ambidentate ligands eg. ![]() and
and ![]() ions. Whereas when a di– or polydentate ligand uses its two or more donor atoms to bind a single metal ion, it is called chelate ligand.
ions. Whereas when a di– or polydentate ligand uses its two or more donor atoms to bind a single metal ion, it is called chelate ligand.
Ans 2.
(i) ![]()
(ii) ![]()
(iii) ![]()
(vi) ![]()
(v) ![]()
(vi) ![]()
Ans 3. (i) Hexaamminecobalt(III) chloride
(ii) Pentaamminechloridocobalt(III) chloride
(iii) Potassium hexacyanoferrate(III)
(iv) Potassium trioxalatoferrate(III)
(v) Potassium tetrachloridopalladate(II)
(vi) Diamminechlorido(methylamine)platinum(II) chloride
Ans 4. Both geometrical (cis-, trans-) isomers for ![]() can exist. Also, optical isomers for cis isomer exist.
can exist. Also, optical isomers for cis isomer exist.
Trans-isomer is optically inactive. On the other hand, cis isomer is optically active.
(ii) Two optical isomers for ![]() exist.
exist.
Two optical isomers are possible for this structure.

(iii) ![]()
A pair of optical isomers:
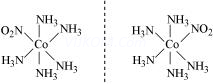
It can also show linkage isomerism.
![]() and
and![]()
It can also show ionization isomerism.
![]()
(iv) Geometrical (cis-, trans-) isomers of ![]() can exist.
can exist.
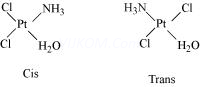
Ans 5. Ni is in the +2-oxidation state i.e., in ![]() configuration.
configuration.
![]()
There are 4 ![]() ions. Thus, it can either have a tetrahedral geometry or square planar geometry. Since
ions. Thus, it can either have a tetrahedral geometry or square planar geometry. Since ![]() ion is a strong field ligand, it causes the pairing of unpaired 3d electrons.
ion is a strong field ligand, it causes the pairing of unpaired 3d electrons.
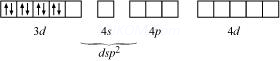
It now undergoes ![]() hybridization. Since all electrons are paired, it is diamagnetic.
hybridization. Since all electrons are paired, it is diamagnetic.
In case of ![]() ,
, ![]() ion is a weak field ligand. Therefore, it does not lead to the pairing of unpaired 3d electrons. Therefore, it undergoes
ion is a weak field ligand. Therefore, it does not lead to the pairing of unpaired 3d electrons. Therefore, it undergoes ![]() hybridization.
hybridization.
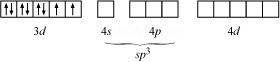
Since there are 2 unpaired electrons in this case, it is paramagnetic in nature.
Ans 6. Though both ![]() and
and ![]() are tetrahedral, their magnetic characters are different. This is due to a difference in the nature of ligands.
are tetrahedral, their magnetic characters are different. This is due to a difference in the nature of ligands. ![]() is a weak field ligand and it does not cause the pairing of unpaired 3d electrons. Hence,
is a weak field ligand and it does not cause the pairing of unpaired 3d electrons. Hence, ![]() is paramagnetic.
is paramagnetic.
![]()
In ![]() , Ni is in the zero-oxidation state i.e., it has a configuration of
, Ni is in the zero-oxidation state i.e., it has a configuration of ![]() .
.
![]()
But CO is a strong field ligand. Therefore, it causes the pairing of unpaired 3d electrons. Also, it causes the 4s electrons to shift to the 3d orbital, thereby giving rise to ![]() hybridization. Since no unpaired electrons are present in this case,
hybridization. Since no unpaired electrons are present in this case, ![]() is diamagnetic.
is diamagnetic.
Ans 7. In both ![]() and
and ![]() , Fe exists in the +3-oxidation state i.e., in
, Fe exists in the +3-oxidation state i.e., in ![]() configuration.
configuration.
![]()
Since ![]() is a strong field ligand, it causes the pairing of unpaired electrons. Therefore, there is only one unpaired electron left in the d-orbital.
is a strong field ligand, it causes the pairing of unpaired electrons. Therefore, there is only one unpaired electron left in the d-orbital.
![]()
Therefore,
![]()
![]()
![]()
=1.732 BM
On the other hand, ![]() is a weak field ligand. Therefore, it cannot cause the pairing of electrons. This means that the number of unpaired electrons is 5.
is a weak field ligand. Therefore, it cannot cause the pairing of electrons. This means that the number of unpaired electrons is 5.
Therefore,
![]()
![]()
![]()
= 6 BM
Thus, it is evident that ![]() is strongly paramagnetic, while
is strongly paramagnetic, while ![]() is weakly paramagnetic.
is weakly paramagnetic.
Ans 8.
|
|
|
|
Oxidation state of cobalt = +3 |
Oxidation state of Ni = +2 |
|
Electronic configuration of cobalt = |
Electronic configuration of nickel = |
|
|
|
Assertion and Reason Answers-
1. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
Explanation:
In these complexes, the metal and ligand fonn a bond that involves the ππ-electrons of the ligand and so it is a ππ-bonded organometallic compound.
2. (d) Assertion is wrong statement but reason is correct statement.
Explanation:
4d and 5d elements have greater tendency to form low spin complex (allows better pairing of electrons) in comparison to 3d because the difference in energy of t2g and eg (CFSE, Δ0) increases in 4d and 5d.
Case Study Answers-
1. Answer :
Explanation:
Oxidation state of Mn in [Mn2(CO)10] is zero.
Explanation:
In [V(CO)6]–, the anionic carbonyl complex can delocalise more electron density to antibonding π−orbital (dπ−pπ back bonding) of CO and thus lowers the bond order.
Explanation:
V(CO)6 can be easily reduced to [V(CO)6]–. V(CO)6 has a total of 17 bonding electrons, hence it is very reactive and unstable. [V(CO)6]– on the other hand has complete set of 18 bonding electrons as an electron is added into the bonding orbital when V(CO)6 gets reduced to [V(CO)6]–. All others have 18 bonding electrons.
Explanation:
K[Co(CO)4]
+1 + (x) + 4(0) = 0 or x = -1
Explanation:
Mn2(CO)10 is made up of two square pyramidal Mn(CO)5 units joined by Mn-Mn bond.
2. Answer :
Explanation:
Ligands are named in alphabetical order irrespective of their charge.
Explanation:
Ligand NO−2 is ambidentate ligand as it can donate electrons through either nitrogen (NO2) or oxygen (ONO).