Important Questions
Multiple Choice questions-
1.The linkage which holds various amino acid units in primary structure of proteins is
2.Vitamin A is called
3.The deficiency of vitamin B1 causes which disease?
4.Deficiency of vitamin C causes
5.An example of non-reducing sugar is
6.Which of the following is not an essential amino acid?
7.Which of the following is a water-soluble vitamin?
8.Vitamin B1 is
9.Which is sweetest of the following:
10.Rickets may be caused by the deficiency of which vitamin?
Very Short Question:
Short Questions:
Long Questions:
Assertion and Reason Questions:
1.In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Uracil occurs in DNA.
Reason: DNA undergoes replication.
2.In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Cysteine can cross link peptide chains.
Reason: Amino acids are classified as essential and non-essential amino acids.
Case Study Questions:
1.Read the passage given below and answer the following questions:
When a protein in its native form, is subjected to physical changes like change in temperature or chemical changes like change in pH, the hydrogen bonds are disturbed. Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein.
The denaturation causes change in secondary and tertiary structures but primary structures remains intact. Examples of denaturation of protein are coagulation of egg white on boiling, curdling of milk, formation of cheese when an acid is added to milk.
The following questions are multiple choice questions. Choose the most appropriate answer:
2.Read the passage given below and answer the following questions:
Carbohydrates are polyhydroxy aldehydes and ketones and those compounds which on hydrolysis give such compounds are also carbohydrates. The carbohydrates which are not hydrolysed are called monosaccharides. Monosaccharides with aldehydic group are called aldose and those which free ketonic groups are called ketose. Carbohydrates are optically active. Number of optical isomers = 2n
Where n = numberofasymmetric carbons. Carbohydrates are mainlysynthesised by plants during photosynthesis. The monosaccharides give the characteristic reactions of alcohols and carbonyl group (aldehydes and ketones). It has been found that these monosaccharides exist in the form of cyclic structures. In cyctization, the -OH groups (generally C5 or C4 in aldohexoses and C5 or C6 in ketohexoses) combine with the aldehyde or keto group. As a result, cyclic structures of five or six membered rings containing one oxygen atom are formed, e.g., glucose forms a ring structure. Glucose contains one aldehyde group, one IO alcoholic group and four 2° alcoholic groups in its open chain structure.
The following questions are multiple choice questions. Choose the most appropriate answer:
Among the above, correct statements are:
Answers key
MCQ answers:
Very Short Answers:
carbohydrates, proteins, Nucleic acids, Lipids, enzymes etc.
|
Monosaccharide |
Disaccharides |
Polysaccharides |
|
Glucose |
Sucrose |
Glycogen |
|
Fructose |
Maltose |
|
|
Ribose |
Lactose |
|
Short Answers:
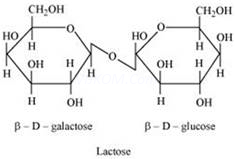
Lactose is composed of ![]() -D-galactose and
-D-galactose and ![]() -D-glucose. Thus, on hydrolysis, it gives
-D-glucose. Thus, on hydrolysis, it gives ![]() -D-galactose
-D-galactose
and ![]() -D-glucose.
-D-glucose.
![]()
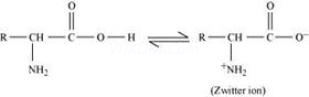
Both acidic (carboxyl) as well as basic (amino) groups are present in the same molecule of amino acids. In aqueous solutions, the carboxyl group can lose a proton and the amino group can accept a proton, thus giving rise to a dipolar ion known as a zwitter ion.
Due to this dipolar behaviour, they have strong electrostatic interactions within them and with water. But halo-acids do not exhibit such dipolar behaviour.
For this reason, the melting points and the solubility of amino acids in water is higher than those of the corresponding halo-acids.
A DNA molecule is double-stranded in which the pairing of bases occurs. Adenine always pairs with thymine, while cytosine always pairs with guanine. Therefore, on hydrolysis of DNA, the quantity of adenine produced is equal to that of thymine and similarly, the quantity of cytosine is equal to that of guanine.
But when RNA is hydrolyzed, there is no relationship among the quantities of the different bases obtained. Hence, RNA is single-stranded.
Monosaccharides are carbohydrates that cannot be hydrolysed further to give simpler units of polyhydroxy aldehyde or ketone.
Monosaccharides are classified on the bases of number of carbon atoms and the functional group present in them. Monosaccharides containing an aldehyde group are known as aldoses and those containing a keto group are known as ketoses. Monosaccharides are further classified as trioses, tetroses, pentoses, hexoses, and heptoses according to the number of carbon atomsthey contain. For example, a ketose containing 3 carbon atoms is called ketotriose and an aldose containing 3 carbon atoms is called aldotriose.
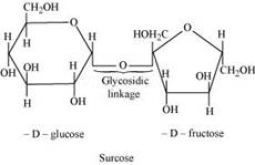
Glycosidic linkage refers to the linkage formed between two monosaccharide units through an oxygen atom by the loss of a water molecule.
For example, in a sucrose molecule, two monosaccharide units, ![]() -glucose and
-glucose and ![]() -fructose, are joined together by a glycosidic linkage.
-fructose, are joined together by a glycosidic linkage.
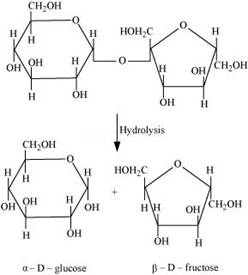
(i) On hydrolysis, sucrose gives one molecule of ![]() -D glucose and one molecule of
-D glucose and one molecule of ![]() -fructose.
-fructose.
(ii) The hydrolysis of lactose gives ![]() -galactose and
-galactose and ![]() -glucose.
-glucose.

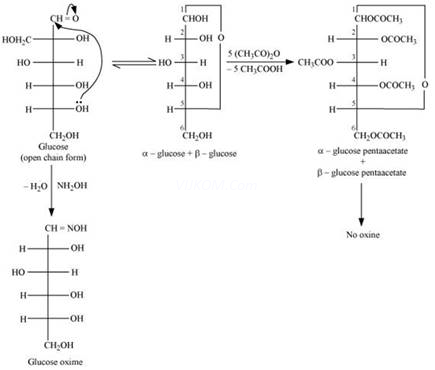
(i) When D-glucose is heated with HI for a long time, n-hexane is formed.
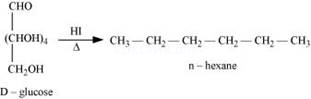
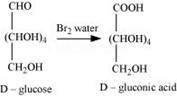
(ii) When D-glucose is treated with ![]() water, D- gluconic acid is produced.
water, D- gluconic acid is produced.
(iii) On being treated with ![]() , D-glucose get oxidised to give saccharic acid.
, D-glucose get oxidised to give saccharic acid.
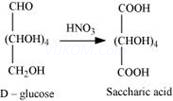
(1) Aldehydes give 2, 4-DNP test, Schiff’s test, and react with ![]() to form the hydrogen sulphite addition product. However, glucose does not undergo these reactions.
to form the hydrogen sulphite addition product. However, glucose does not undergo these reactions.
(2) The pentaacetate of glucose does not react with hydroxylamine. This indicates that a free -CHO group is absent from glucose.
(3) Glucose exists in two crystalline forms – ![]() and
and ![]() . The
. The![]() form (m.p. = 419 K) crystallises from a concentrated solution of glucose at 303 K and the
form (m.p. = 419 K) crystallises from a concentrated solution of glucose at 303 K and the ![]() form (m.p = 423 K) crystallises from a hot and saturated aqueous solution at 371 K. This behavior cannot be explained by the open chain structure of glucose.
form (m.p = 423 K) crystallises from a hot and saturated aqueous solution at 371 K. This behavior cannot be explained by the open chain structure of glucose.
I n aqueous solution, the carboxyl group of an amino acid can lose a proton and the amino group can accept a proton to give a dipolar ion known as zwitter ion.
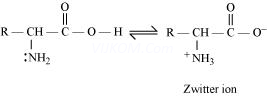
Therefore, in zwitter ionic form, the amino acid can act both as an acid and as a base.
![]()

Thus, amino acids show amphoteric behaviour.
|
Fibrous protein |
Globular protein |
||
|
1. |
It is a fibre-like structure formed by the polypeptide chain. These proteins are held together by strong hydrogen and disulphide bonds. |
1. |
The polypeptide chain in this protein is folded around itself, giving rise to a spherical structure. |
|
2. |
It is usually insoluble in water. |
2. |
It is usually soluble in water. |
|
3. |
Fibrous proteins are usually used for structural purposes. For example, keratin is present in nails and hair; collagen in tendons; and myosin in muscles. |
3. |
All enzymes are globular proteins. Some hormones such as insulin are also globular proteins. |
Long Answers:
D-glucose reacts with hydroxylamine(NH2OH) to form an oxime because of the presence of aldehydic (-CHO) group or carbonyl carbon. This happens as the cyclic structure of glucose forms an open chain structure in an aqueous medium, which then reacts with NH2OH to give an oxime.

But pentaacetate of D-glucose does not react with NH2OH. This is because pentaacetate does not form an open chain structure.
Starch consists of two components – amylose and amylopectin. Amylose is a long linear chain of∝-D-(+)-glucose units joined by C1-C4 glycosidic linkage (∝–link).
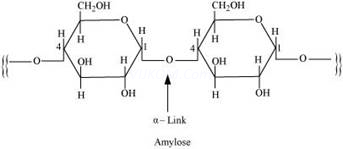
Amylopectin is a branched-chain polymer of ∝-D-glucose units, in which the chain is formed by C1-C4 glycosidic linkage and the branching occurs by C1-C6 glycosidic linkage.
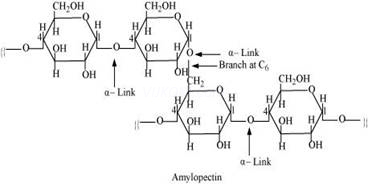
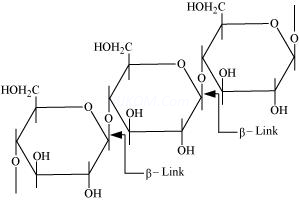
On the other hand, cellulose is a straight-chain polysaccharide ofβ -D-glucose units joined by C1-C4 glycosidic linkage (β-link).
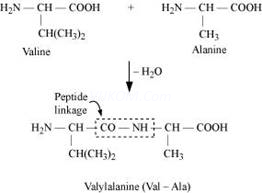
(i)Peptide linkage:
The amide formed between -COOH group of one molecule of an amino acid and -NH2 group of another molecule of the amino acid by the elimination of a water molecule is called a peptide linkage.
(ii) Primary structure:
The primary structure of protein refers to the specific sequence in which various amino acids are present in it, i.e., the sequence of linkages between amino acids in a polypeptide chain. The sequence in which amino acids are arranged is different in each protein. A change in the sequence creates a different protein.
(iii) Denaturation:
In a biological system, a protein is found to have a unique 3-dimensional structure and a unique biological activity. In such a situation, the protein is called native protein. However, when the native protein is subjected to physical changes such as change in temperature or chemical changes such as change in pH, its H-bonds are disturbed. This disturbance unfolds the globules and uncoils the helix. As a result, the protein loses its biological activity. This loss of biological activity by the protein is called denaturation. During denaturation, the secondary and the tertiary structures of the protein get destroyed, but the primary structure remains unaltered.
One of the examples of denaturation of proteins is the coagulation of egg white when an egg is boiled.
4. Answer:
There are two common types of secondary structure of proteins:
(i) ![]() -helix structure
-helix structure
(ii) ![]() pleated sheet structure
pleated sheet structure
![]() – Helix structure:
– Helix structure:
In this structure, the -NH group of an amino acid residue forms H-bond with the ![]() group of the adjacent turn of the right-handed screw (
group of the adjacent turn of the right-handed screw (![]() -helix).
-helix).
![]() pleated sheet structure:
pleated sheet structure:
This structure is called so because it looks like the pleated folds of drapery. In this structure, all the peptide chains are stretched out to nearly the maximum extension and then laid side by side. These peptide chains are held together by intermolecular hydrogen bonds.
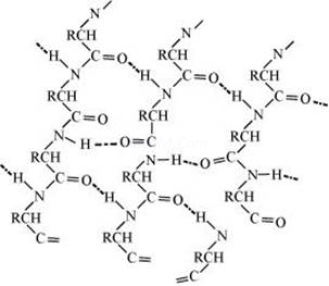
5.Answer:
The structural differences between DNA and RNA are as follows:
The functional differences between DNA and RNA are as follows:
Assertion and Reason Answers:
1. (d) Assertion is wrong statement but reason is correct statement.
Explanation:
Uracil occurs in RNA
2. (b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
Explanation:
Cysteine can cross link peptide chains through disulphide bridge. Cross linking by disulphide bridge can occur either between the distant, properly oriented parts of the same polypeptide chain (as in oxytocin or vasopressin) or between different polypeptide chains.
Case Study Answers:
1. Answer :
Explanation:
Cheese is a denatured protein.
2. Answer :
Explanation:
Glucose contains four chiral centres.
Explanation:
In the formation of osazone, C-1 and C-2 react with phenylhydrazine to form phenylhydrazone. If C-3, C-4, C-5 have same configuration they will form same osazone even if they differ in configuration at C-1 or C-2.
Explanation:
Pentacetate of glucose does not react with hydroxylamine showing absence of free -CHO group. This cannot be explained by open structure of glucose.
|
DNA |
RNA |
||
|
1. |
The sugar moiety in DNA molecules is β -D-2 deoxyribose. |
1. |
The sugar moiety in RNA molecules is β -D-ribose. |
|
2. |
DNA contains thymine (T). It does not contain uracil (U). |
2. |
RNA contains uracil (U). It does not contain thymine (T). |
|
3. |
The helical structure of DNA is double – stranded. |
3. |
The helical structure of RNA is single-stranded. |
|
DNA |
RNA |
||
|
1 |
DNA is the chemical basis of heredity. |
1 |
RNA is not responsible for heredity. |
|
2 |
DNA molecules do not synthesise proteins, but transfer coded message for the synthesis of proteins in the cells. |
2 |
Proteins are synthesised by RNA molecules in the cells. |


