Important Questions
Multiple Choice questions-
Question 1.Which of the following does not react with Hinsberg reagent?
(a) Ethylamine
(b) (CH3)2NH
(c) (CH3)3N
(d) Propan-2-amine
Question 2.![]()
in above sequence, Z is
(a) Cyanoethane
(b) Ethanamide
(c) Methanamine
(d) Ethanamine
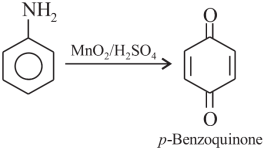
Question 3.Oxidation of aniline with K2Cr2O7/H2SO4 gives
(a) phenylhydroxylamine
(b) p-benzoquinone
(c) nitrosobenzene
(d) nitrobenzene
Question 4.Which of the following amines can exhibit enantiomerism?
(a) Benzeamine
(b) 2-Butanamine
(c) 2-Propanamine
(d) 2-Methyl-propanamine.
Question 5.Which of the following: when heated with a mixture of ethanmine and alcoholic potash gives ethyl isocyanide?
(a) 2-chloropropane
(b) 2,2-dichloropropane
(c) trichloromethane
(d) tetrachloromethane
Question 6.Which of the following pair of species will yield carbylamine?
(a) CH3CH2Br and KCN
(b) CH3CH2Br and NH3 (excess)
(c) CH3CH2Br and AgCN
(d) CH3CH2NH2 and HCHO
Question 7.Which one of the following methods is neither meant for the synthesis nor for separation of amines?
(a) Hinsberg method
(b) Hoffmann method
(c) Wurtz reaction
(d) Curticus reaction
Question 8.C6H5CONHCH3 can be converted into C6H5CH2NHCH3 by
(a) NaBH4
(b) H2-Pd/C
(c) LiAlH4
(d) Zn-Hg/HCl
Question 9.C6H5N+2 Cl– + CuCN → C6H5CN + N2 + CuCl. The above chemical reaction is associated with which of the following name:
(a) Balz Schiemen
(b) Gattermann
(c) Shimonini
(d) Sandmeyer.
Question 10.The reaction of aniline with benzoyl chloride gives
(a) Benzoin
(b) Benzanilide
(c) Benzalaniline
(d) Benzamide
Very Short Questions–
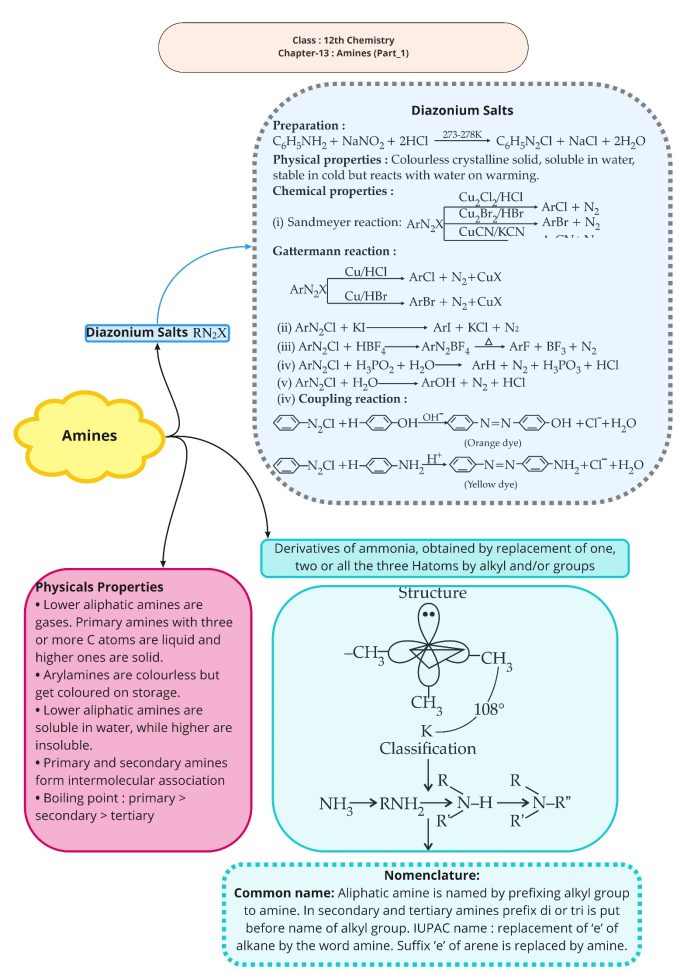
1.For an amine RNH2, write an expression to indicate its basic strength.
2. Give one use of quaternary ammonium salts.
3. Give one example of Hoffmann – Bromamide reaction
4. Distinguish between ethylamine and aniline.
5.The IUPAC name of secondary amine having lowest molecular mass is _________.
6.Give an example of diazotization
7. Write one use of diazonium salt
8.How can the reactivity of aromatic amines be controlled?
9.Give one use of tertiary amines.
10. Name a reagent which can distinguish between primary, secondary and tertiary amine
Short Questions—
1. It is difficult to prepare pure amines by ammonolysis of alkylhalides.
2. Amines have higher boiling points than hydrocarbons of similar molecular mass.
3. Aniline is weaker base than cyclohexylamine.
4. Methylamine is a stronger base than aniline.
5. Before nitration, aniline is converted to acetanilide.
6. It is easier to brominate aniline as compared to benzene.
7. Reduction of nitro compound to aniline using iron scrap and HCl is preferred.
8. Aromatic amines cannot be prepared by Gabriel Phthalimide synthesis.
9. During acylation of amines, pyridine is added.
10. Aniline does not undergo Friedel – Craft’s reaction.
Long Questions–
1. (i) Write structures of different isomeric amines corresponding to the molecular formula, ![]()
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
2. Arrange the following in increasing order of their basic strength:
(i) ![]() and
and![]()
(ii) ![]()
(iii) ![]() .
.
3. Write structures of different isomers corresponding to the molecular formula, ![]() . Write IUPAC names of the isomers which will liberate nitrogen gas on treatment with nitrous acid.
. Write IUPAC names of the isomers which will liberate nitrogen gas on treatment with nitrous acid.
4. Write IUPAC names of the following compounds and classify them into primary, secondary and tertiary amines.
(i) ![]() (ii)
(ii) ![]()
(iii) ![]() (iv)
(iv) ![]()
(v) ![]() (vi)
(vi) ![]() (vii)
(vii) ![]()
5. Give one chemical test to distinguish between the following pairs of compounds.
(i) Methylamine and dimethylamine
(ii) Secondary and tertiary amines
(iii) Ethylamine and aniline
(iv) Aniline and benzylamine
(v) Aniline and N-methylaniline.
6. Account for the following:
(i) pKb of aniline is more than that of methylamine.
(ii) Ethylamine is soluble in water whereas aniline is not.
(iii) Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide.
(iv) Although amino group is o, p- directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
(v) Aniline does not undergo Friedel-Crafts reaction.
(vi) Diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(vii) Gabriel phthalimide synthesis is preferred for synthesising primary amines.
7. Arrange the following:
(i) In decreasing order of the pKbvalues:
![]()
![]() and
and ![]()
(ii) In increasing order of basic strength:
![]() ,
, ![]() ,
, ![]() and
and ![]()
(iii) In increasing order of basic strength:
(a) Aniline, p-nitroaniline and p-toluidine
(b) ![]() ,
, ![]() ,
, ![]() .
.
(iv) In decreasing order of basic strength in gas phase:
![]() , (C2H5)2NH,
, (C2H5)2NH, ![]() and
and ![]()
(v) In increasing order of boiling point:
![]() ,
, ![]() ,
, ![]()
(vi) In increasing order of solubility in water:
![]() ,
, ![]() ,
, ![]() .
.
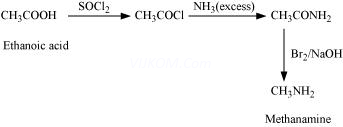
8. How will you convert:
(i) Ethanoic acid into methanamine
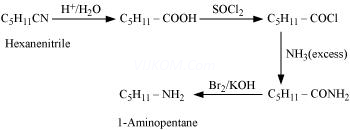
(ii) Hexanenitrile into 1-aminopentane
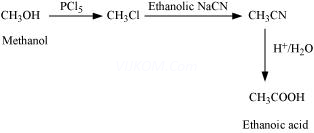
(iii) Methanol to ethanoic acid
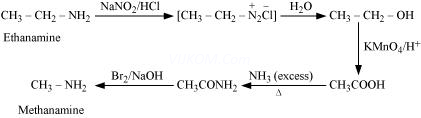
(iv) Ethanamine into methanamine
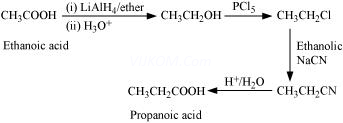
(v) Ethanoic acid into propanoic acid
(vi) Methanamine into ethanamine
(vii) Nitromethane into dimethylamine
(viii) Propanoic acid into ethanoic acid
Assertion and Reason Questions–
1.In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Ortho substituted anilines are usually weaker bases than anilines.
Reason: This is due to ortho effect.
2.In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
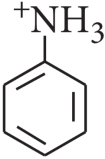
Assertion: In strongly acidic solutions, aniline becomes more reactive towards electrophilic reagents.
Reason: The amino group being completely protonated in strongly acidic solution, the lone pair of electrons on the nitrogen is no longer available for resonance.
Case Study Questions–
1.Read the passage given below and answer the following questions:
Amines are alkyl or aryl derivatives of ammonia formed by replacement of one or more hydrogen atoms. Alkyl derivatives are called aliphatic amines and aryl derivatives are known as aromatic amines. The presence of aromatic amines can be identified by performing dye test. Aniline is the simplest example of aromatic amine. It undergoeselectrophilic substitution reactions in which -NH2 group strongly activates the aromatic ring through delocalisation of lone pair of electrons of N-atom. Aniline undergoes electrophilic substitution reactions. Ortho and para positions to the -NH2 group become centres of high electrons density. Thus, -NH2 group is ortho and para-directing and powerful activating group.
The following questions are multiple choice questions. Choose the most appropriate answer:
2.Read the passage given below and answer the following questions:
The amines are basic in nature due to the presence of a lone pair of electron on N-atom of the -NH2 group, which it can donate to electron deficient compounds. Aliphatic amines are stronger bases than NH3 because of the +I effect of the alkyl groups. Greater the number of alkyl groups attached to N-atom, higher is the electron density on it and more will be the basicity. Thus, the order of basic nature of amines is expected to be 3°> 2°> 1°, however the observed order is 2°> 1°> 3°. This is explained on the basis of crowding on N-atom of the amine by alkyl groups which hinders the approach and bonding by a proton, consequently, the electron pair which is present on N is unavailable for donation and hence 3° amines are the weakest bases.
Aromatic amines are weaker bases than ammonia and aliphatic amines. Electron-donating groups such as -CH3, -OCH3, etc. increase the basicity while electron-withdrawing substitutes such as -NO2, -CN, halogens, etc. decrease the basicity of amines. The effect of these substituents is more at p than at m-positions.
The following questions are multiple choice questions. Choose the most appropriate answer:
MCQ Answers–
Very Short Answers–
Ans 1.
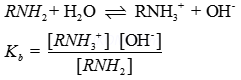
Ans 2. Quaternary ammonium salts are widely used for manufacture of synthetic detergents.
Ans 3. In Hoffmann – Bromamide reaction an acid amide is reacted with Bromine in presence of a base to give a primary amine having one carbon less than the starting amide.
![]()
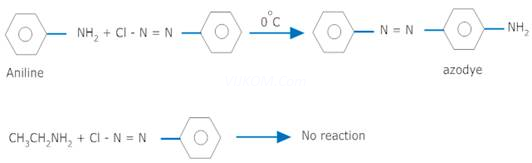
Ans 4. Ethylamine and aniline can be distinguished by azodye test. On treating aniline with benzene diazonium salt, orange or red coloured azodye is formed which is not formed with ethyl amine.
Ans 5. N- Methylmethanamine.
Ans 6. During diazotization benzene diazonium chloride is prepared by the reaction of aniline with nitrous acid at 273 – 278 K

Ans 7.Diazonium salts are used in preparation of substituted aromatic compounds.
Ans 8.The reactivity of aromatic amines can be controlled by acylation.
Ans 9.Tertiary amines like trimethylamine are used as insect attractants.
Ans 10. P- Toluenesulphonyl chloride Hinsberg reagent can be used as a distinguishing reagent for primary, secondary and tertiary amines.
Short Answers–
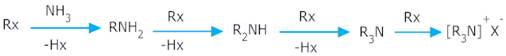
Ans 1. The process of ammonolysis yields a mixture of primary, secondary, tertiary amines and quaternary salts. The separation of this amines is a very complicated process and difficult. Therefore it is difficult to prepare pure amines by ammonolysis of alkyl halides.
Ans 2. Amines have higher boiling points than hydrocarbons of comparable molecular mass due to the presence of intermolecular hydrogen bond in amines which is absent in hydrocarbons. Therefore, amines exist as associated molecules and have higher boiling points.
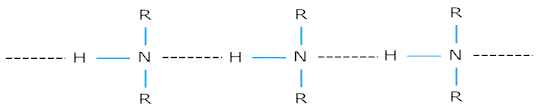
In alcohols and carboxylic acids, the electro- negativity of oxygen is more than nitrogen of amines. Therefore the hydrogen bonds of alcohols and acids are stronger than in amines and alcohols & carboxylic acids have higher boiling points.
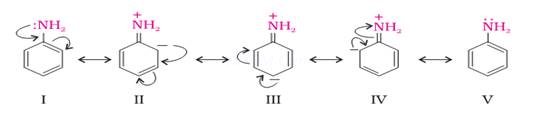
Ans 3. As a result of resonance in aniline; the lone pair on nitrogen delocalized over the benzene ring and is less available for protonation than in cyclohexyl amine which does not undergo resonance.
Resonating structure of aniline –
Ans 4. Due to electron donating nature of![]() , group, electron pair. Availability at N- atom in methyl amine is much higher than that in aniline; in aniline the benzene ring decreases the electron density at N- atom in aniline. Thus
, group, electron pair. Availability at N- atom in methyl amine is much higher than that in aniline; in aniline the benzene ring decreases the electron density at N- atom in aniline. Thus ![]() is a stronger base than aniline.
is a stronger base than aniline.
Ans 5. Aniline is very much susceptible to nitration and nitric acid is a strong oxidizing agent. Therefore to avoid oxidation of aniline, ![]() group is protected by its acetylation to acetanilide which undergo nitration smoothly without any oxidation.
group is protected by its acetylation to acetanilide which undergo nitration smoothly without any oxidation.
Ans 6. In aniline, due to +R effect of ![]() group the benzene ring gets activated to a large extent and it becomes easier to brominates aniline as compared to benzene.
group the benzene ring gets activated to a large extent and it becomes easier to brominates aniline as compared to benzene.
Ans 7. For reduction of nitro compounds to aniline, iron scrap and HCl is preferred because ![]() formed gets hydrolysed to release HCl during the reaction & therefore only a small amount of HCl is required to initiate the reaction.
formed gets hydrolysed to release HCl during the reaction & therefore only a small amount of HCl is required to initiate the reaction.
Ans 8. Aromatic amines cannot be prepared by Gabriel pythalimide synthesis as aryl halides do not undergo nucleophilic substitution with the anion formed by pythalimide.
Ans 9. Acylation of amines is carried out in presence of pyridine or another base stronger than amines as it removes HCl so formed and shifts the equilibrium in forward direction.

Ans 10. During Fridel Craft’s reaction, aniline forms salt with aluminum chloride, the catalyst of reaction due to which nitrogen acquires a positive charge and acts as a strong deactivating group for further reaction.
Long Answers-
Ans 1. (i), (ii) The structures and their IUPAC names of different isomeric amines corresponding to the molecular formula, ![]() are given below:
are given below:
(a)![]()
Butanamine![]()
(b) 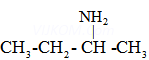
Butan-2-amine ![]()
(c) 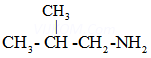
2-Methylpropanamine ![]()
(d) 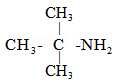
2-Methylpropan-2-amine ![]()
(e) ![]()
N-Methylpropanamine![]()
(f) ![]()
N-Ethylethanamine![]()
(g) ![]()
N-Methylpropan-2-amine ![]()
(h) ![]()
N, N-Dimethylethanamine![]()
(iii) The pairs (a) and (b) and (e) and (g) exhibit position isomerism.
The pairs (a) and (c); (a) and (d); (b) and (c); (b) and (d) exhibit chain isomerism.
The pairs (e) and (f) and (f) and (g) exhibit metamerism.
All primary amines exhibit functional isomerism with secondary and tertiary amines and vice-versa.
Ans 2. (i) Considering the inductive effect of alkyl groups, ![]() and
and ![]() can be arranged in the increasing order of their basic strengths as:
can be arranged in the increasing order of their basic strengths as:
![]()
Again, ![]() has proton acceptability less than
has proton acceptability less than ![]() . Thus, we have:
. Thus, we have:
![]()
Due to the – I effect of ![]() group, the electron density on the N-atom in
group, the electron density on the N-atom in ![]() is lower than that on the N-atom in
is lower than that on the N-atom in ![]() , but more than that in
, but more than that in ![]() . Therefore, the given compounds can be arranged in the order of their basic strengths as:
. Therefore, the given compounds can be arranged in the order of their basic strengths as:
![]()
(ii) Considering the inductive effect and the steric hindrance of the alkyl groups, ![]() and their basic strengths as follows:
and their basic strengths as follows:
![]()
Again, due to the – R effect of ![]() group, the electron density on the N atom in
group, the electron density on the N atom in ![]() is lower than that on the N atom in
is lower than that on the N atom in ![]() . Therefore, the basicity of
. Therefore, the basicity of ![]() is lower than that of
is lower than that of ![]() . Hence, the given compounds can be arranged in the increasing order of their basic strengths as follows:
. Hence, the given compounds can be arranged in the increasing order of their basic strengths as follows:
![]()
(iii) Considering the inductive effect and the steric hindrance of alkyl groups, ![]() , and .
, and .![]() .can be arranged in the increasing order of their basic strengths as:
.can be arranged in the increasing order of their basic strengths as:
![]()
In ![]() , N is directly attached to the benzene ring. Thus, the lone pair of electrons on the N – atom is delocalized over the benzene ring. In
, N is directly attached to the benzene ring. Thus, the lone pair of electrons on the N – atom is delocalized over the benzene ring. In ![]() , N is not directly attached to the benzene ring. Thus, its lone pair is not delocalized over the benzene ring. Therefore, the electrons on the N atom are more easily available for protonation in
, N is not directly attached to the benzene ring. Thus, its lone pair is not delocalized over the benzene ring. Therefore, the electrons on the N atom are more easily available for protonation in ![]() than in
than in ![]() i.e.,
i.e., ![]() is more basic than
is more basic than ![]() .
.
Again, due to the – I effect of ![]() group, the electron density on the N – atom in
group, the electron density on the N – atom in ![]() is lower than that on the N – atom in
is lower than that on the N – atom in ![]() . Therefore,
. Therefore, ![]() is more basic than
is more basic than ![]() . Thus, the given compounds can be arranged in the increasing order of their basic strengths as follows.
. Thus, the given compounds can be arranged in the increasing order of their basic strengths as follows.
![]()
Ans 3. The structures of different isomers corresponding to the molecular formula, ![]() are given below:
are given below:
(a) ![]()
Propan-1-amine ![]()
(b)
![]()
Propan-2-amine ![]()
(c)
![]()
(d)
![]()
N, N-Dimethylmethanamine ![]()
![]() amines, (a) propan-1-amine, and (b) Propan-2-amine will liberate nitrogen gas on treatment with nitrous acid.
amines, (a) propan-1-amine, and (b) Propan-2-amine will liberate nitrogen gas on treatment with nitrous acid.
![]()
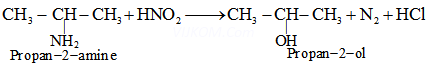
Ans 4. (i) 1-Methylethanamine (![]() amine)
amine)
(ii) Propan-1-amine (![]() amine)
amine)
(iii) N-Methyl-2-methylethanamine (![]() amine)
amine)
(iv) 2-Methylpropan-2-amine (![]() amine)
amine)
(v) N-Methylbenzamine or N-methylaniline (![]() amine)
amine)
(vi) N-Ethyl-N-methylethanamine (![]() amine)
amine)
(vii) 3-Bromobenzenamine or 3-bromoaniline (![]() amine)
amine)
Ans 5. (i) Methylamine and dimethylamine can be distinguished by the carbylamine test.
Carbylamine test: Aliphatic and aromatic primary amines on heating with chloroform and ethanolic potassium hydroxide form foul-smelling isocyanides or carbylamines. Methylamine (being an aliphatic primary amine) gives a positive carbylamine test, but dimethylamine does not.
(ii) Secondary and tertiary amines can be distinguished by allowing them to react with Hinsberg’s reagent (benzenesulphonyl chloride, ![]() ).
).
Secondary amines react with Hinsberg’s reagent to form a product that is insoluble in an alkali. For example, N, N – diethylamine reacts with Hinsberg’s reagent to form N, N – diethylbenzenesulphonamide, which is insoluble in an alkali. Tertiary amines, however, do not react with Hinsberg’s reagent.
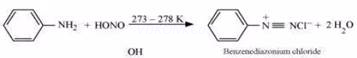
(iii) Ethylamine and aniline can be distinguished using the azo-dye test. A dye is obtained when aromatic amines react with ![]() at
at ![]() , followed by a reaction with the alkaline solution of 2-naphthol. The dye is usually yellow, red, or orange in colour. Aliphatic amines give a brisk
, followed by a reaction with the alkaline solution of 2-naphthol. The dye is usually yellow, red, or orange in colour. Aliphatic amines give a brisk
effervescence due (to the evolution of ![]() gas) under similar conditions.
gas) under similar conditions.
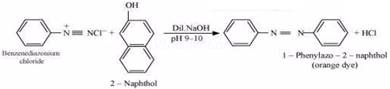
![]()
(iv) Aniline and benzylamine can be distinguished by their reactions with the help of nitrous acid, which is prepared in situ from a mineral acid and sodium nitrite. Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas.
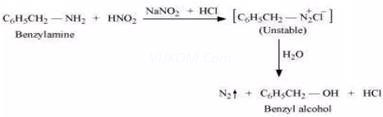
On the other hand, aniline reacts with ![]() at a low temperature to form stable diazonium salt. Thus, nitrogen gas is not evolved.
at a low temperature to form stable diazonium salt. Thus, nitrogen gas is not evolved.
![]()
(v) Aniline and N-methylaniline can be distinguished using the Carbylamine test. Primary amines, on heating with chloroform and ethanolic potassium hydroxide, form foul-smelling isocyanides or carbylamines. Aniline, being an aromatic primary amine, gives positive carbylamine test. However, N-methylaniline, being a secondary amine does not.
Ans 6. (i) pKbof aniline is more than that of methylamine:

Aniline undergoes resonance and as a result, the electrons on the N-atom are delocalized over the benzene ring. Therefore, the electrons on the N-atom are less available to donate.
On the other hand, in case of methylamine (due to the +I effect of methyl group), the electron density on the N-atom is increased. As a result, aniline is less basic than methylamine. Thus, pKb of aniline is more than that of methylamine.
(ii)Ethylamine is soluble in water whereas aniline is not:
Ethylamine when added to water forms intermolecular H – bonds with water. Hence, it is soluble in water.
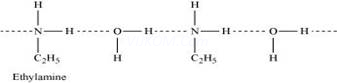
But aniline does not undergo H – bonding with water to a very large extent due to the presence of a large hydrophobic – C6H5 group. Hence, aniline is insoluble in water.
![]()
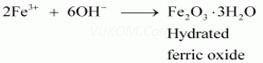
(iii)Methylamine in water reacts with ferric chloride to precipitate hydrated ferric oxide:
![]()
Due to the +I effect of – ![]() group, methylamine is more basic than water. Therefore, in water, methylamine produces OH – ions by accepting H+ ions from water.
group, methylamine is more basic than water. Therefore, in water, methylamine produces OH – ions by accepting H+ ions from water.
![]()
Ferric chloride ![]() dissociates in water to form
dissociates in water to form ![]() ions.
ions.
![]()
Then, OH – ion reacts with Fe3+ ion to form a precipitate of hydrated ferric oxide.
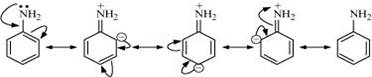
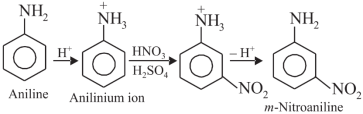
(iv)Although amino group is o, p – directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline:
Nitration is carried out in an acidic medium. In an acidic medium, aniline is protonated to give anilinium ion (which is meta-directing).
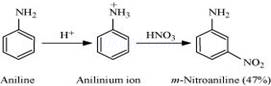
For this reason, aniline on nitration gives a substantial amount of m-nitroaniline.
(v)Aniline does not undergo Friedel-Crafts reaction:
A Friedel-Crafts reaction is carried out in the presence of ![]() . But
. But ![]() is acidic in nature, while aniline is a strong base. Thus, aniline reacts with
is acidic in nature, while aniline is a strong base. Thus, aniline reacts with ![]() to form a salt (as shown in the following equation).
to form a salt (as shown in the following equation).
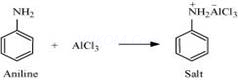
Due to the positive charge on the N-atom, electrophilic substitution in the benzene ring is deactivated. Hence, aniline does not undergo the Friedel-Crafts reaction.
(vi)Diazonium salts of aromatic amines are more stable than those of aliphatic amines:
The diazonium ion undergoes resonance as shown below:
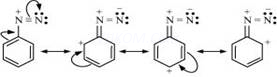
This resonance accounts for the stability of the diazonium ion. Hence, diazonium salts of aromatic amines are more stable than those of aliphatic amines.
(vii)Gabriel phthalimide synthesis is preferred for synthesising primary amines:
Gabriel phthalimide synthesis results in the formation of amine only. ![]() or
or ![]() amines are not formed in this synthesis. Thus, a pure
amines are not formed in this synthesis. Thus, a pure ![]() amine can be obtained. Therefore, Gabriel phthalimide synthesis is preferred for synthesizing primary amines.
amine can be obtained. Therefore, Gabriel phthalimide synthesis is preferred for synthesizing primary amines.
Ans 7. (i) In![]() , only one –
, only one – ![]() group is present while in
group is present while in![]() , two –
, two – ![]() groups are present. Thus, the +I effect is more in
groups are present. Thus, the +I effect is more in ![]() than in
than in![]() . Therefore, the electron density over the N-atom is more in
. Therefore, the electron density over the N-atom is more in ![]() than in
than in![]() . Hence,
. Hence, ![]() is more basic than
is more basic than![]() .
.
Also, both ![]() and
and ![]() are less basic than
are less basic than ![]() and
and ![]() due to the delocalization of the lone pair in the former two. Further, among
due to the delocalization of the lone pair in the former two. Further, among ![]() and
and ![]() , the former will be more basic due to the +T effect of –
, the former will be more basic due to the +T effect of – ![]() group. Hence, the order of increasing basicity of the given compounds is as follows:
group. Hence, the order of increasing basicity of the given compounds is as follows:
![]() <
< ![]() <
<![]() <
< ![]()
We know that the higher the basic strength, the lower is the pKb values.
![]() >
> ![]() >
> ![]() >
> ![]()
(ii) ![]() is more basic than
is more basic than ![]() due to the presence of the +I effect of two -CH3 groups in
due to the presence of the +I effect of two -CH3 groups in ![]() . Further,
. Further, ![]() contains one –
contains one – ![]() group while
group while ![]() contains two –
contains two – ![]() groups. Thus,
groups. Thus, ![]() is more basic than
is more basic than![]() .
.
Now, ![]() is less basic than CH3NH2 because of the-R effect of –
is less basic than CH3NH2 because of the-R effect of – ![]() group.
group.
Hence, the increasing order of the basic strengths of the given compounds is as follows:
![]() <
< ![]() <
< ![]() <
< ![]()
(iii) (a)
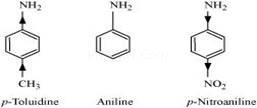
In p-toluidine, the presence of electron-donating –![]() group increases the electron density on the N-atom.
group increases the electron density on the N-atom.
Thus, p-toluidine is more basic than aniline.
On the other hand, the presence of electron-withdrawing
–![]() group decreases the electron density over the N-atom in p-nitroaniline. Thus, p-nitroaniline is less basic than aniline.
group decreases the electron density over the N-atom in p-nitroaniline. Thus, p-nitroaniline is less basic than aniline.
Hence, the increasing order of the basic strengths of the given compounds is as follows:
p-Nitroaniline< Aniline <p-Toluidine
(b) ![]() is more basic than
is more basic than ![]() due to the presence of electron-donating –
due to the presence of electron-donating –![]() group in
group in ![]() .
.
Again, in![]() , –
, –![]() group is directly attached to the N-atom. However, it is not so in
group is directly attached to the N-atom. However, it is not so in![]() . Thus, in
. Thus, in ![]() , the -R effect of –
, the -R effect of –![]() group decreases the electron density over the N-atom. Therefore,
group decreases the electron density over the N-atom. Therefore, ![]() is more basic than
is more basic than ![]() .
.
Hence, the increasing order of the basic strengths of the given compounds is as follows:
![]() <
< ![]() <
<![]() .
.
(iv) In the gas phase, there is no solvation effect. As a result, the basic strength mainly depends upon the +I effect. The higher the +I effect, the stronger is the base. Also, the greater the number of alkyl groups, the higher is the +I effect. Therefore, the given compounds can be arranged in the decreasing order of their basic strengths in the gas phase as follows:
![]() >
> ![]() >
> ![]() >
> ![]()
(v) The boiling points of compounds depend on the extent of H-bonding present in that compound. The more extensive the H-bonding in the compound, the higher is the boiling point. ![]() contains only one H-atom whereas
contains only one H-atom whereas ![]() contains two H-atoms. Then,
contains two H-atoms. Then, ![]() undergoes more extensive H-bonding than
undergoes more extensive H-bonding than ![]() . Hence, the boiling point of
. Hence, the boiling point of ![]() is higher than that of
is higher than that of![]() .
.
Further, O is more electronegative than N. Thus, ![]() forms stronger H-bonds than
forms stronger H-bonds than ![]() . As a result, the boiling point of
. As a result, the boiling point of ![]() is higher than that of
is higher than that of ![]() and
and ![]() .
.
Now, the given compounds can be arranged in the increasing order of their boiling points as follows:
![]() <
< ![]() <
< ![]()
(vi) The more extensive the H-bonding, the higher is the solubility. ![]() contains two H-atoms whereas
contains two H-atoms whereas ![]() contains only one H-atom. Thus,
contains only one H-atom. Thus, ![]() undergoes more extensive H-bonding than
undergoes more extensive H-bonding than ![]() . Hence, the solubility in water of
. Hence, the solubility in water of ![]() is more than that of
is more than that of ![]() .
.
Further, the solubility of amines decreases with increase in the molecular mass. This is because the molecular mass of amines increases with an increase in the size of the hydrophobic part. The molecular mass of ![]() is greater than that of
is greater than that of ![]() and
and ![]() .
.
Hence, the increasing order of their solubility in water is as follows:
![]() <
< ![]() <
< ![]()
Ans 8. (i)
(ii)
(iii)
(iv)
(v)
(vi)
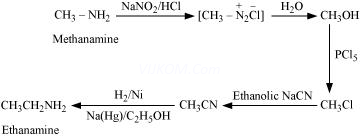
(vii)
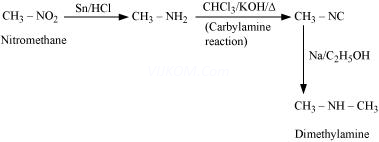
(viii)
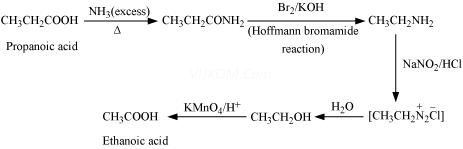
Assertion and Reason Answers–
1. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
Explanation:
Ortho effect is a consequence of steric and electronic factors.
2.(d) Assertion is wrong statement but reason is correct statement.
Explanation:
In strongly acidic medium, aniline gets protonated and so the lone pair of electrons is not available to produce +E or + M effects. On the other hand, the −N+H3−N+H3 group exerts strong -I effect and thus it causes the deactivation of the ring.
Case Study Answers–
1. Answer :
Explanation:
Aromatic primary amines give dye test.
Explanation:
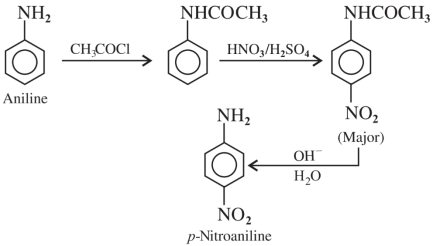
Explanation:
Explanation:
In acidic medium aniline gets protonated to anilinium ion which is meta-directing.
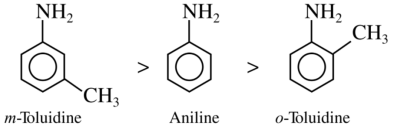
2. Answer :
Explanation:
The increasing order of basicity of the given compounds is (CH3)2NH > CH3NH2 > (CH3)3N > C6H5NH2. Due to the +I effect of alkyl groups, the electron density on nitrogen increases and thus, the availability of the lone pair of electrons to proton increases and hence, the basicity of amines also increases. So, aliphatic amines are more basic than aniline. ln case of tertiary amine (CH3)3N, the covering of alkyl groups over nitrogen atom from all sides makes the approach and bonding by a proton relatively difficult, hence the basicity decreases. Electron withdrawing groups decrease electron density on nitrogen atom and thereby decreasing basicity.
Explanation:
In general, electron donating ( +R) group which when present on benzene ring (-NH2, -OR, -R, etc.) at the para position increases the basicity of aniline.
Ortho substituted anilines are weaker bases than aniline due to ortho effect.
Explanation:
In case of ethylamines, the combined effect of (c) inductive effect, steric effect and salvation effect gives the order of basic strength as
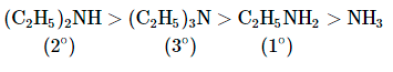
Explanation:
In non-aqueous solvents the basic strength of alkyl amines follows the order:
tertiary amines> secondary amines> primary amines.
Explanation:
Methyl amine is stronger base than ammonia due to electron releasing inductive effect of methyl group.




