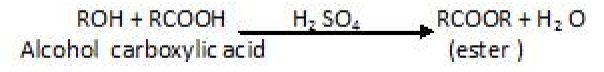ALDEHYDES, KETONES, AND CARBOXYLIC ACIDS
Introduction
 (WhereR=Horalkylorarylgroup)
(WhereR=Horalkylorarylgroup)
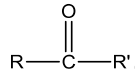 (WhereRandR’maybesameordifferentalkylorarylgroups)
(WhereRandR’maybesameordifferentalkylorarylgroups)
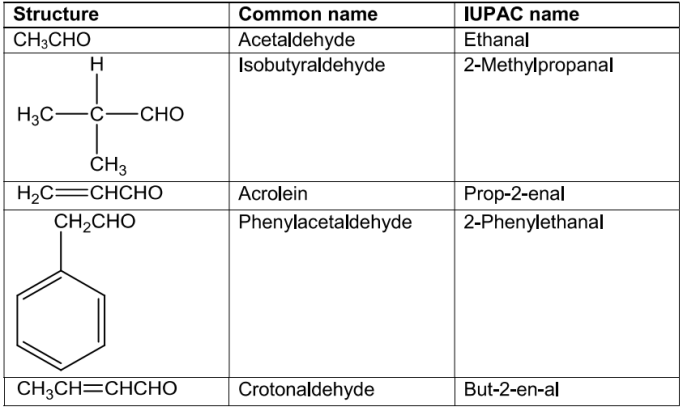
NomenclatureofAldehydesandKetones
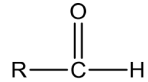
ldehydes
![]()
![]()
![]()
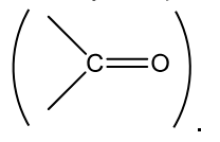
Ketones
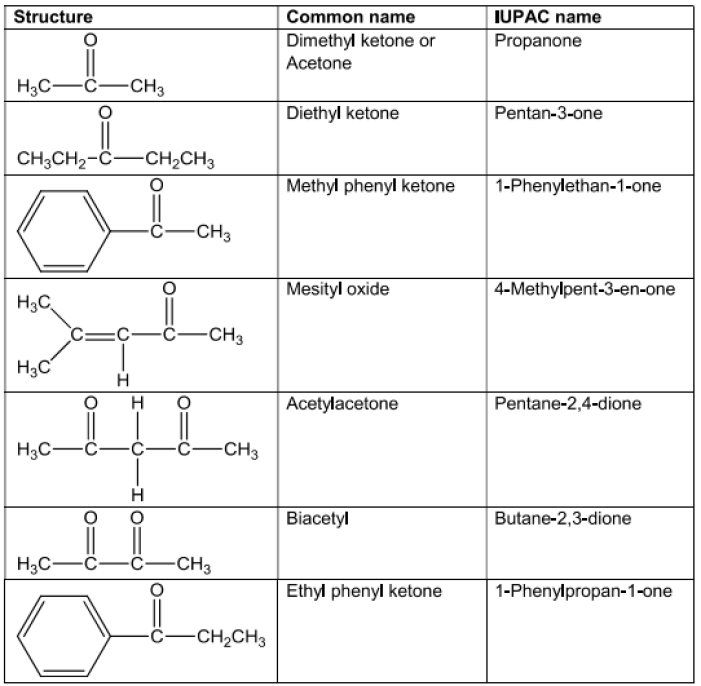
StructureandNatureofCarbonylGroup
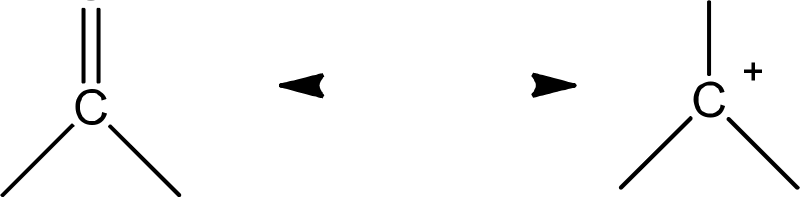
Structure
-+
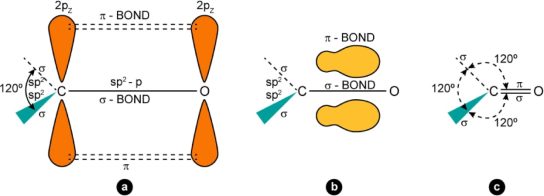
Nature
(A)andadipolar(B)structuresgivenbelow.
 O O
O O
PreparationofAldehydes
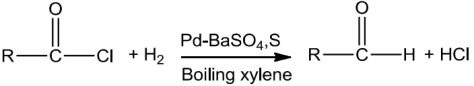
RosenmundReduction
In this reaction, acyl chloride on hydrogenation in the presence of palladium catalyst and bariumsulphategivesaldehydes.
Nitriles onreductionwithstannouschlorideinthepresenceofHCIgiveiminewhichonhydrolysisgivescorrespondingaldehyde.
![]()
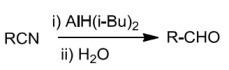
An alternate method to reduce nitriles selectively is by diisobutylaluminium hydride to imines which onhydrolysisyieldsaldehydes.
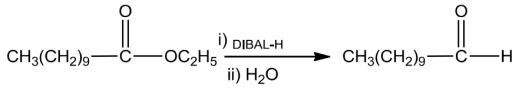
EsterscanalsobereducedtoaldehydeswithDIBAL-H
Aromatic aldehydescanbeprepared usingthefollowingmethods.
EtardReaction(UseofChromylChloride)
Chromylchlorideoxidisesthemethylgrouptoachromiumcomplexwhichonfurther hydrolysisgivescorrespondingbenzaldehyde.
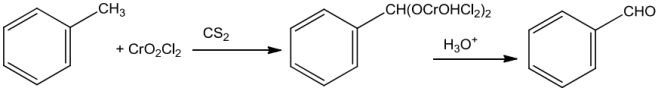
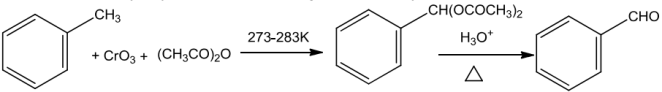
UseofChromicoxide(CrO3)
Toluene when treated with chromic oxide in acetic anhydride gets converted into benzylidenediacetatewhichonhydrolysiswithaqueousacid givesbenzaldehyde.
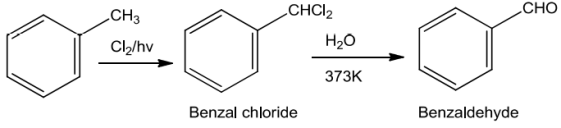
SideChainChlorination
Tolueneonsidechlorinationgivesbenzalchloridewhichonhydrolysisgivesbenzaldehyde.
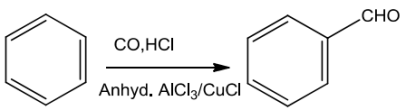
Gatterman—KochReaction
BenzeneortolueneontreatmentwithCOandHCIinthepresence ofAIC!3orCuCIgivesbenzaldehydeorp-tolualdehyde.
Preparation of Ketones
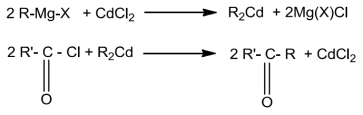
From Acid chlorides or Acyl chlorides
AcylchlorideontreatmentwithdialkylcadmiumobtainedbyreactionofcadiumchloridewithGrignardreagentgivesketones.
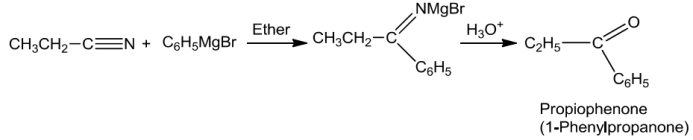
NitrilesontreatmentwithGrignardreagentfollowedbyhydrolysisyieldsaketone
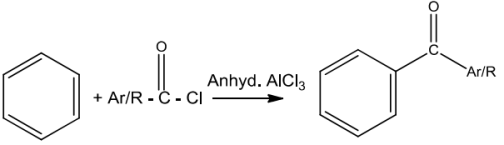
BenzeneorsubstitutedbenzeneontreatmentwithacidchlorideinthepresenceofanhydrousAICI3givesthecorrespondingketoneandthisreactionisknownasFriedel-Craftsacylationreaction.
ChemicalReactions
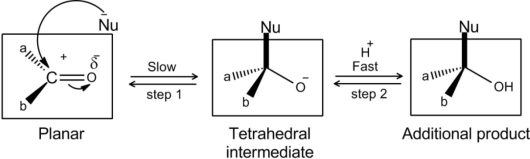
AldehydesandKetones undergonucleophilicadditionreactions.
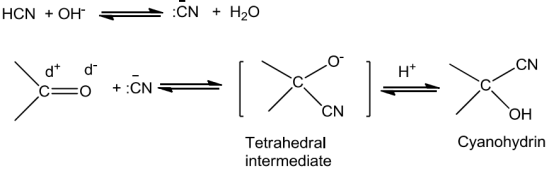
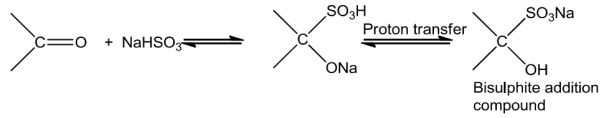
Sodiumhydrogensulphitewhenaddedtoaldehydesandketonesyieldadditionproducts.
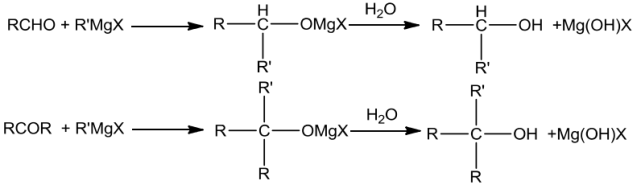
Grignard reagentsonreactingwithaldehydesandketones yieldalcohols.

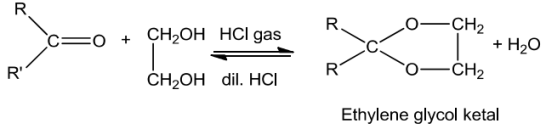
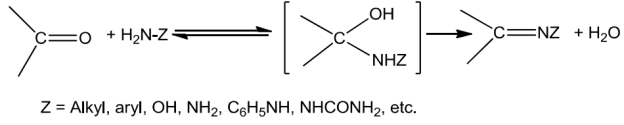
Ammoniaanditsderivativeaddtothecarbonylgroupofanaldehydesandketone
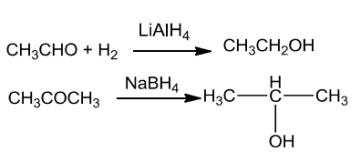
AldehydesandketonesgetreducedtoprimaryandsecondaryalcoholsbyNaBH4orLiAIH4.
Aldehydes and ketones reduce to —CH2group on treatment with zinc-amalgam and conc.HCI[CIemmenson reduction] or with hydrazine which on heating with sodium or potassiumhydroxideinethyleneglycol[Wolff-Kishnerreduction]
![]()
TesttodistinguishAldehydesfromKetones
Tollenstest
![]()
Fehling’stest
FehlingsolutionB=Alkalinesodium potassiumtartarate(Rochellesalt)
Haloformreaction
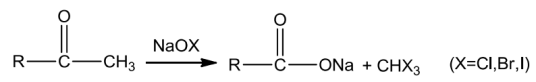
Aldehydes and ketones with at least one methyl group attached to the carbonyl carbon atom on oxidation with sodium hypohalite turn to sodium salts of corresponding acids with one carbon atom less than that of the carbonyl compound.In this reaction, the methyl group is converted to haloform.
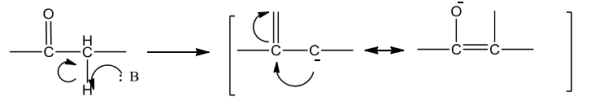
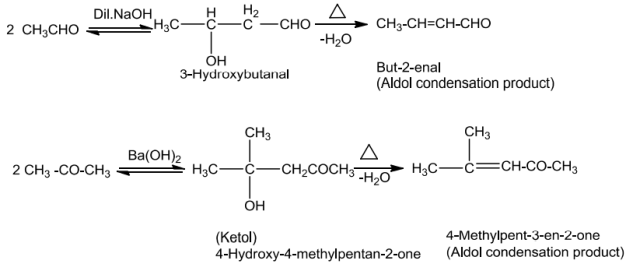
Aldehydes and ketones with at least one a-hydrogen undergo reaction in the presence of dilutealkali as catalyst to form §-hydroxy aldehydes (aldol) or §-hydroxy ketones (ketol) respectively.ThisisknownasAldolreaction.
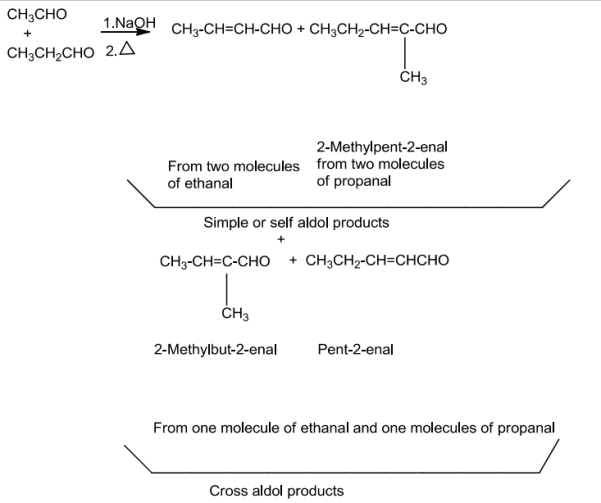
Ketones can be taken as one the component in the cross aldol reactions.
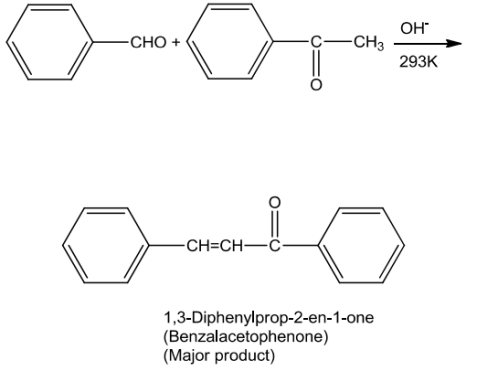
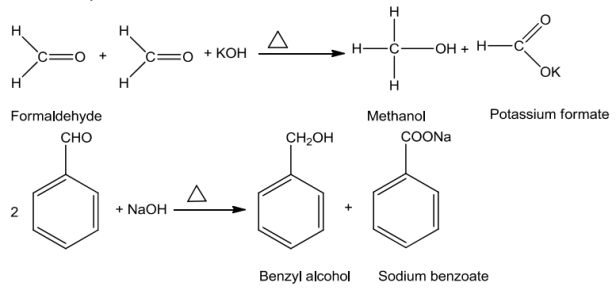
Aromatic aldehydes and ketones undergo electrophilic substitution reaction at the ring in which the carbonyl group acts as an activating and meta-directing group.
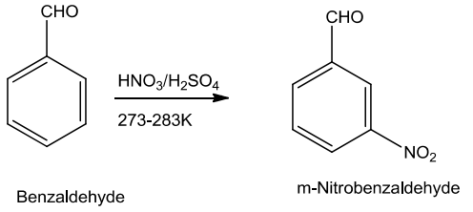
Carboxylic Acids
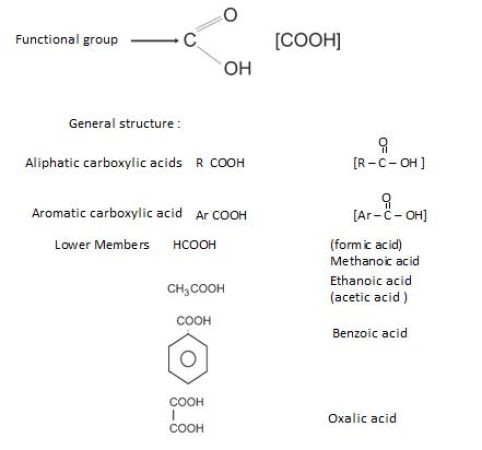
NomenclatureofCarbonylGroup
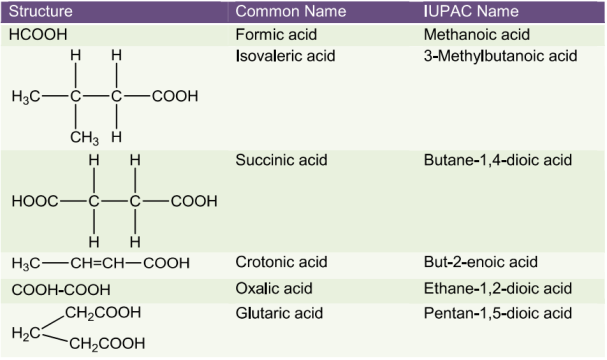
StructureofCarbonylGroup
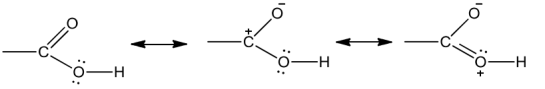
The bonds to the carboxyl carbon in carboxylic acids lie in one plane and are separated by about 120°.Due to possible resonance structure given below, the carboxylic carbon is less electrophilic than carbonyl carbon.
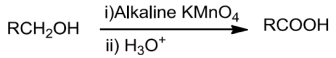
PreparationofCarboxylicAcids
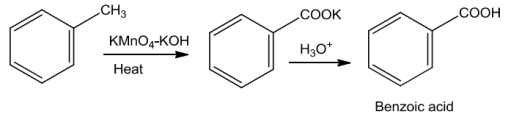
![]()
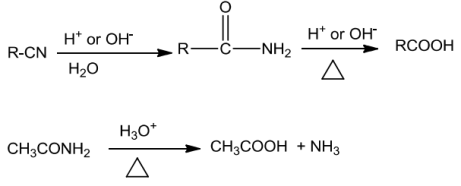
![]()
Grignard reagentsontreatingwithcarbondioxideformsaltsofcarboxylicacidswhichonacidificationwithmineralacidgivecorrespondingcarboxylicacids.
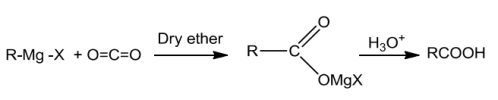
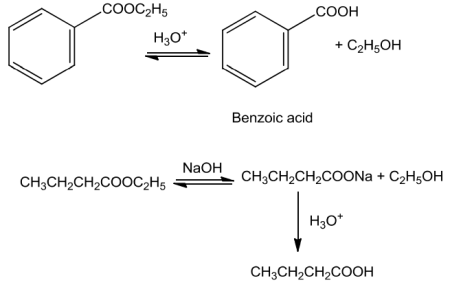
Esters on hydrolysis with acid give acids directly while basic hydrolysis give carboxylates which onacidificationgivecorrespondingacids.
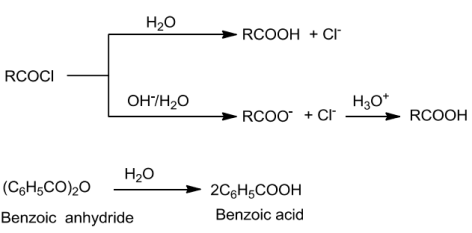
ChemicalReactionsofCarboxylicAcids
ReactionsinvolvingcleavageofO-Hbond
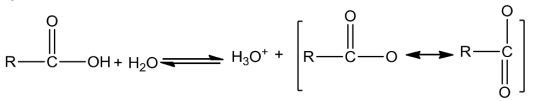
Acidity of carboxylic acids:Carboxylic acids are more acidic than phenols. The strength ofaciddependsontheextentofionisation, which in turn depends on the stability of the anion formed.
Theeffectofthefollowinggroupsinincreasingacidityorderis:
Ph<I<Br< CI<F<CN<NO2<CF3
Anhydridesareobtained ontreatingcarboxylicacidswithmineralacidssuchasH2SO4orwith
P2O5.
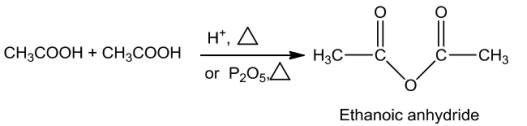
Esters are formed on treating alcohols or phenols with carboxylic acids in the presence of conc.H2SO4orHCIgasasacatalyst.
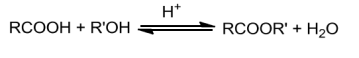
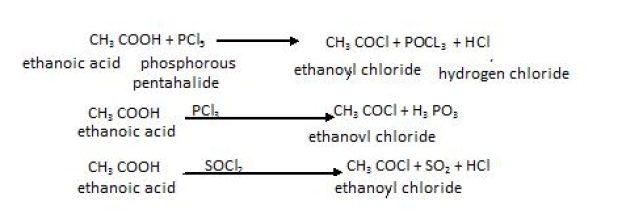
OntreatingwithPCI5,PCl3orSOCl2,thehydroxylgroupofalcoholsisreplacedbychlorineatom.SOCI2is preferred since the two products formed are volatile and escape easily making thepurificationoftheproductseasier.
RCOOH+PCl5→RCOCI+POCI3+ HCI3RCOOH+PCI3→3RCOCI + H3PO3RCOOH+SOCl2→RCOCI+SO2+ HCI
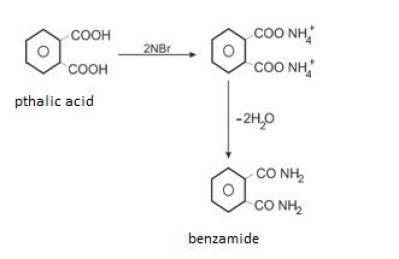
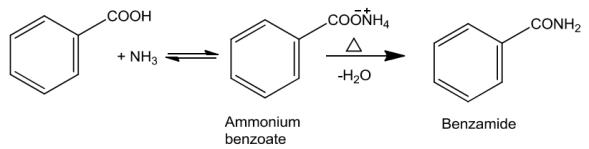
When carboxylic acids are allowed to react with ammonia, ammonium salt is formed which onfurtherheatingathightemperaturegivesamides.
![]()
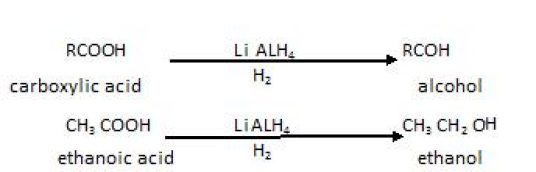
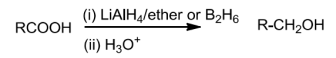
OnreductionwithreducingagentslikeLiAIH4ordiborane,carboxylicacidsarereduced toprimary alcohols.
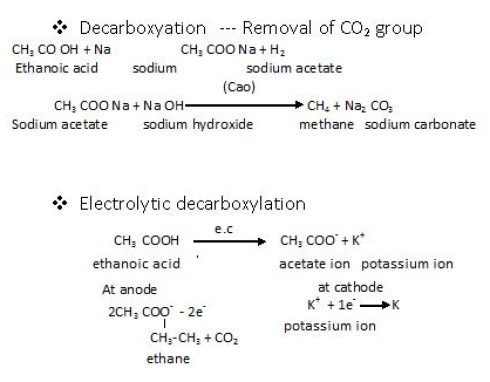
Sodium salts of carboxylic acids on heating with sodalime lose carbon dioxide to formhydrocarbons.Thereactionis knownasdecarboxylation.
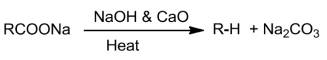
SubstitutionreactionsintheHydrocarbon
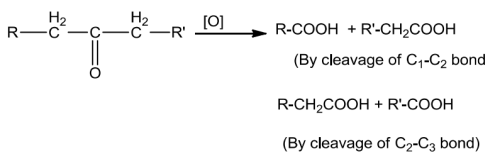
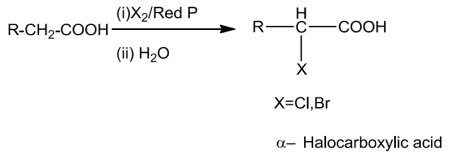
Carboxylic acids with a-hydrogen atom undergo halogenation at the a-position on treatment withsmall amount of red phosphorus to give a-halocarboxylic acids. The reaction is known as HeII-Volhard-Zelinskyreaction.
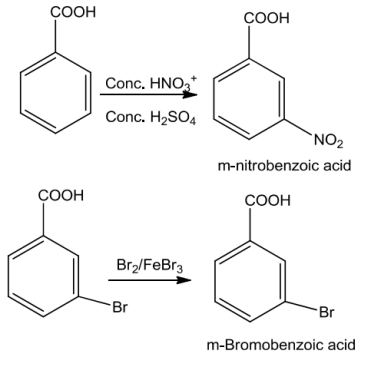
Aromaticcarboxylicacidsundergoelectrophilicsubstitutionreactionsinwhichthecarboxyl groupactsasadeactivatingandmeta-directinggroup.
They however do not undergo Friedel-Crafts reaction because the carboxyl group is deactivatingandthecatalystaluminiumchloride(Lewisacid)gets bondedtothe carboxylgroup.
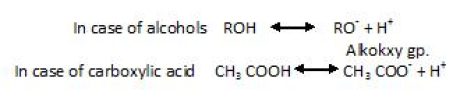
Comparison of acidic strength of alcohol , phenols and carboxylic acid
If we compare Alcohol and carboxylic acid, we see that :
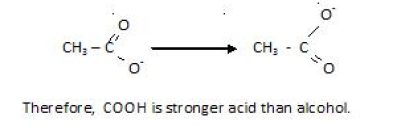
It’s seen that more the ion is stablized , more the reaction will be favoured in found direction.Below you can see carboxylic acid is resonance stabilized. So, it is more stable and on the other hand the R group attached to O- in alcohol intensifies its charge. Hence, the stability is lowered in mparison to carboxylic acid.
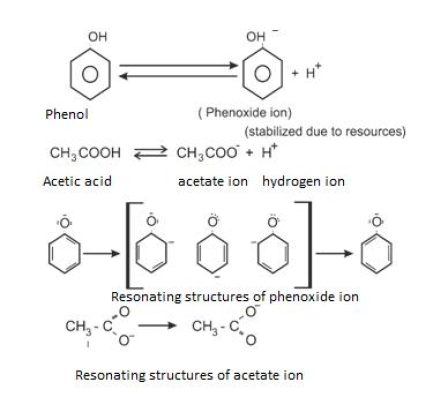
Phenols and carboxylic acid : Acidic character
Similarly, if we look for phenoxide ion and carboxylate ion that is formed by phenol and carboxylic acid after loosing hydrogen ion, we can easily make out that carboxylate ion is more stabilized. In it negative charge resides on electronegative ion (resonating structure ) whereas in case of phenoxide ion the negative charge is on carbon.let’s see. the structures below :
That is the reason ,carboxylic acid is stronger than phenols .
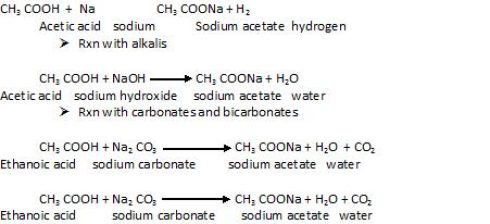
Reactions to prove acidic character of carboxylic acid
Reaction with metal
Reaction involving OH group:
In this we react carboxylic acid with compounds like With PCl5, PCl3, SOCl2.
Reaction with alcohol : Esterification
Reaction with NH3
Formation of acid anhydride
3.Reaction involving COOH group
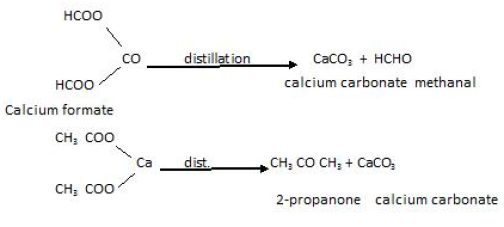
Reaction of carboxylic salt of Calcium
Partial Reduction
The acids on reduction in presence of reducing agents like are Li AlH4 etc forms alcohols that is :
Complete reduction : When complete reduction is carried out, it forms alkane in presence of red Phosphorous that is :
Special name reactions
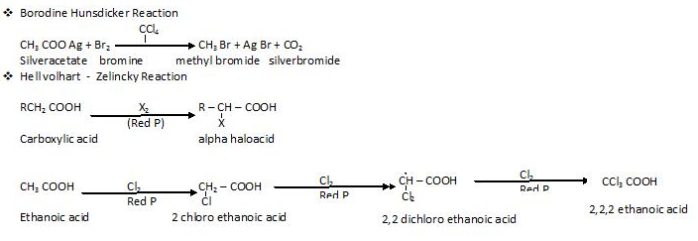
Ring subsitution reactions :
Bromination
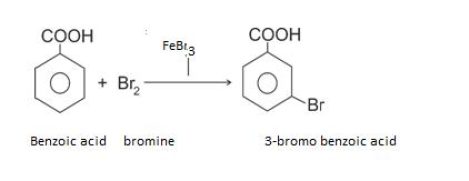
Sulphonation
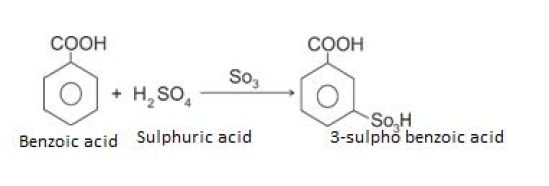
Nitration
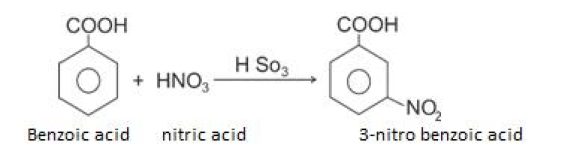
Uses of carboxylic acid
Methanoic acid is used as coagulating agent in rubber industry.
Benzoic acid is used as antiseptic and also in perfumery.
Ethanoic acid is used as solvent and also in cooking as vinegar and much more.
Reaction involving COOH group
Important Questions
Multiple Choice questions-
Question 1. Which of the following cannot reduce Fehling’s solution?
(a) Formic acid
(b) Acetic acid
(c) Formaldehyde
(d) Acetaldehyde
Question 2. Which of the following acids does not form anhydride?
(a) Formic add
(b) Acetic acid
(c) Propionic add
(d) n-butyric acid
Question 3. The acid which does not contain-COOH group is.
(a) Ethanoic acid
(b) Lactic acid
(c) Picric add
(d) Palmitic acid
Question 4. Trans-esterification is a reaction between
(a) two ester molecules
(b) alcohol and carboxylic acid
(c) alcohol and ether
(d) alcohol and ester.
Question 5. Acetone on heating with ammonia produces
(a) Acetaldehyde
(b) Diacetone alcohol
(c) Diacetoneamine
(d) Hydrobenzamide
Question 6. Methyl ketones are usually characterised through
(a) Tollen’s reagent
(b) Iodoform test
(c) Schiff’stest
(d) Benedict solution test.
Question 7. Which of the following reagents can be used to prepare ketone from acid chloride?
(a) Grignard’s reagent
(b) LiAlH4
(c) Dimethyl cadmium
(d) Cadmium chloride
Question 8. HVZ reaction is used to prepare
(a) ß-haloacid
(b) α-haloacid
(c) α, ß-unsaturated add
(d) None of these
Question 9.An alkene C7H14 on reductive ozonolysis gives an aldehyde with formula C3H6O and and a ketone. The ketone is
(a) 2-butanone
(b) 2-pentanone
(c) 3-pentanone
(d) propanone
Question 10. Acetaldol is a condensation product of
(a) two molecules of ethanal
(b) two molecules of propanone
(c) ethanal and methanal
(d) ethanal and propanone.
Very Short Questions-
1. Give one use of Formalin.
2. What is the chemical name of Tollen’s reagent and Fehling’s solution.
3. Write the structure of alkenes that on ozonolysis will give ketone only.
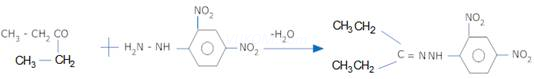
4. What is the function of ![]() in rosenmund reaction?
in rosenmund reaction?
5. Name the isomers with molecular formula![]() . Which one will have high boiling point?
. Which one will have high boiling point?
6. Write a chemical test to distinguish between aldehyde and ketone.
7. What happens when acetaldehyde is kept with a trace of sulphuric acid? Write the structure of product.
8. What is the Hofmann bromamide reaction? Illustrate with one example.
9. Give IUPAC name of following
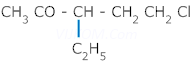
10. Give IUPAC name of following
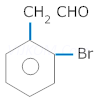
Short Questions-
1. Ethanoic acid has molar mass of 120 in vapour state.
2. Carboxylic acids do not give characteristic reactions of Carboxylic acid is stronger acid than phenol.
3. Ethanol is more soluble in water than ethyl chloride
4. Aldehydes are more reactive than Ketones towards nucleophilic additions.
5. Carboxylic acids has higher boiling points than alcohols of same no. of carbon atoms.
6. carbonyl group.
7. Formaldehyde does not undergo aldol condensation.
8. Floro acetic acid is a stronger acid than acetic acid.
9. Toluene to benzaldehyde
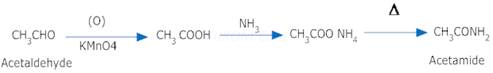
10. Acetaldehyde to Acetamide
Long Questions-
1. A compound ‘A’ with formula ![]() gives a positive 2, 4 –DNP test but a negative Tollen’s test It can be oxidizing to carboxylic acid ‘B’ of molecular formula
gives a positive 2, 4 –DNP test but a negative Tollen’s test It can be oxidizing to carboxylic acid ‘B’ of molecular formula![]() , when treated with alk.
, when treated with alk. ![]() under vigorous conditions. The salt of ‘B’ gives a hydrocarbon ‘C’ on Kolbes’ electrolytic decarboxylation. Identify A, B.C & write chemical equations.
under vigorous conditions. The salt of ‘B’ gives a hydrocarbon ‘C’ on Kolbes’ electrolytic decarboxylation. Identify A, B.C & write chemical equations.
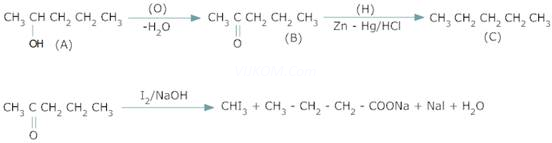
2. Acompound A with molecular formula ![]() on oxidation forms compound B with molecular formula
on oxidation forms compound B with molecular formula![]() . The compound B gives iodoform test but does not reduce ammoniacal silver nitrate. The compound B on reduction with Zn – Hg/ HCl gives compound C with molecular formula
. The compound B gives iodoform test but does not reduce ammoniacal silver nitrate. The compound B on reduction with Zn – Hg/ HCl gives compound C with molecular formula![]() . Identify A,B.C & give the chemical reactions involved.
. Identify A,B.C & give the chemical reactions involved.
3. An organic compound A, which has a characteristic odour, on treatment with NaOH forms two compound B and C. Compound B has molecular formula ![]() which on oxidation gives back A. Compound C is the sodium salt of an acid. C, when heated with soda lime yields an aromatic hydrocarbon D. deduce the structures of A to D.
which on oxidation gives back A. Compound C is the sodium salt of an acid. C, when heated with soda lime yields an aromatic hydrocarbon D. deduce the structures of A to D.
4. ![]()
5.
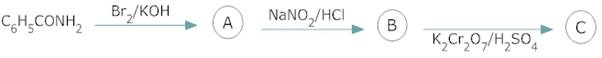
6.
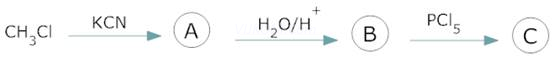
7. ![]()
8. ![]()
Assertion and Reason Questions-
1. In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Aromatic aldehydes and formaldehyde undergo Cannizzaro reaction.
Reason: Aromatic aldehydes are almost as reactive as formaldehyde.
2. In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: O-Substituted benzoic acids are generally stronger acids than benzoic acids.
Reason: Increased strength is due to ortho-effect.
Case Study Questions-
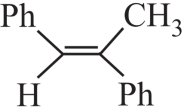
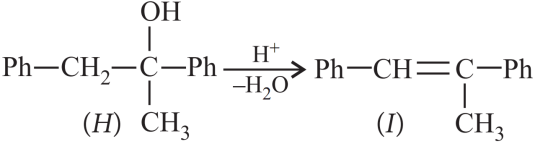
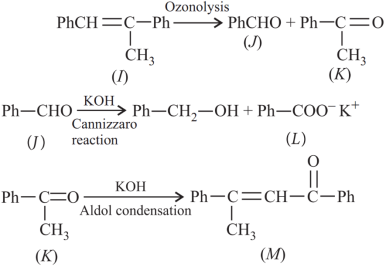
1. Read the passage given below and answer the following questions:
A tertiary alcohol H upon acid catalysed dehydration gives a product I. Ozonolysis of I leads to compounds J and K. Compound J upon reaction with KOH gives benzyl alcohol and a compound I, whereas K on reaction with KOH gives only M.
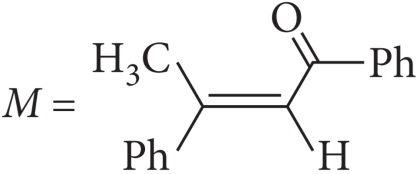
The following questions are multiple choice questions. Choose the most appropriate answer:
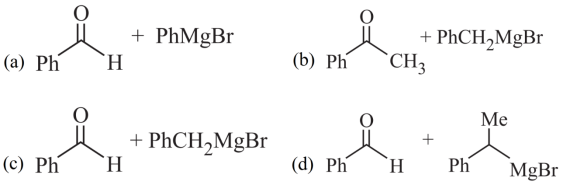
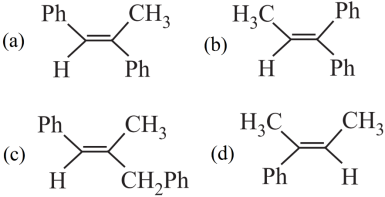
2. Read the passage given below and answer the following questions:
When an aldehyde with no et-hydrogen reacts with concentrated aqueous NaOH, half the aldehyde is converted to carboxylic acid salt and other half is converted to an alcohol. In other words, half of the reactant is oxidized and other half is reduced. This reaction is known as Cannizzaro reaction.
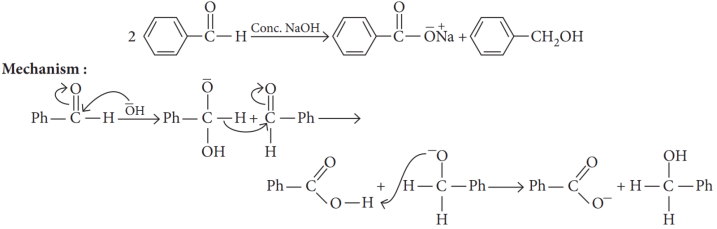
The following questions are multiple choice questions. Choose the most appropriate answer:
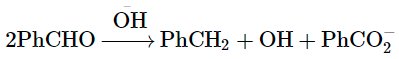
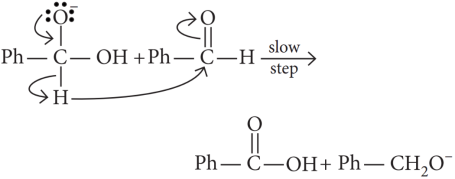
the slowest step is:
MCQ Answers-
Very Short Answers-
Ans 1. Formalin is used as a disinfectant, preservative for biological specimens and in leather industry.
Ans 2. Tollen’s reagent = Ammoniacal Silver Nitrate
Fehlings solution = Sodium Potassium Tartarate.
Ans 3.
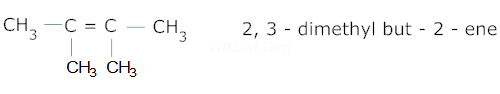
Ans 4. ![]() acts as a catalytic poison which prevents further reduction of aldehyde to alcohol.
acts as a catalytic poison which prevents further reduction of aldehyde to alcohol.
Ans 5. The two isomers are ![]() and
and![]() . Acetone boils at higher temperature due to presence of two electron donating alkyl groups.
. Acetone boils at higher temperature due to presence of two electron donating alkyl groups.
Ans 6. Aldehydes and ketones can be distinguished by Tollen’s test. Aldehydes give a silver mirror on reacting with Tollen’s reagent whereas ketones will not react.
Ans 7. A trimer of acetaldehyde, called paraldchyde is formed.
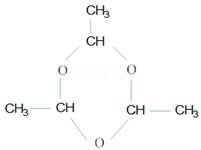
Ans 8. Hoffman bromamide reaction is a reaction in which amides are converted to amines of one carbon less than the starting amide. It is a very important step – down reaction.
![]()
Ans 9. 5-Chloro -3- ethylpentan -2-one.
Ans 10. 2 –(2-bromophenyl) ethanal
Short Answers-
Ans 1. Carboxylic acid on dissociation form carboxylate ion which is stabilized by two equivalent resonance structure in which negative charge is at the more electronegative oxygen atom, whereas the conjugate base of phenol, phenoxide ion, has non – equivalent resonance structures in which negative charge is at the less electronegative carbon atom. Therefore resonance is not as important as it is in carboxylate ion. Moreover the negative charge is delocalized over two more electronegative oxygen atoms in carboxylate ion whereas it is less effectively delocalized over one oxygen atom and one carbon atom in phenoxide ion. Therefore the carboxylate ion is more stabilized than phenoxide ion and carboxylic acids are stronger acids than phenol.
Ans 2. Ethanol can form intermolecular Hydrogen bonding with water molecules, ethyl chloride can not. Therefore ethanol is soluble in water and ethyl chloride is not.
Ans 3. Aldehydes are more reactive than Ketones due to steric and electronic reasons. In Ketones due to presence of two relatively large alkyl groups, the approach of nucleophile is more hindered than in aldehydes having only one such substitute. More over the +I effect of alkyl groups reduces the electophilicity of carbonyl group more in Ketone than in aldehydes.
Ans 4. Carboxylic acids have more extensive association of molecules through intermolecular hydrogen bonding than alcohols. Moreover their boiling points are higher than alcohols of same carbon atoms.
Ans 5. Ethanoic acid exists as dimer in vapour state in which two molecules remain together by hydrogen bonding. This increases the effective molecular mass to 120.
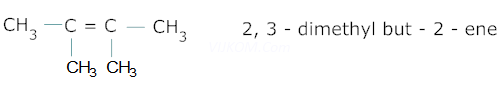
Ans 6. In carboxylic acids due to presence of resonance, the C=O group is not a pure carbonyl group & therefore they do not show characteristic reactions of carbonyl group.
Ans 7. Formaldehyde does not have any ![]() – hydrogen and therefore it can not show aldol condensation.
– hydrogen and therefore it can not show aldol condensation.
Ans 8. In fluoroacetic acid, Fluorine being electron withdrawing group stabilizes the conjugate base through delocalization of the negative charge
![]()
Therefore fluoroacetic acid is a stronger acid than acetic acid.
Ans 9.

Ans 10.
Long Answers-
Ans 1.
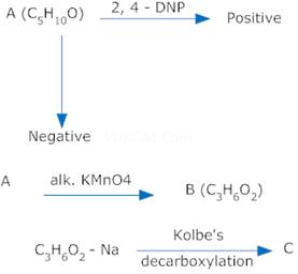
As the compound A gives a positive 2, 4-DNP test but negative Tollen’s test, it is a ketone. Since on oxidation, it gives an acid B, of molecular formula![]() , it is
, it is ![]() and B is
and B is![]() . As C is obtained by Kolbes decarboxylation of B, C is
. As C is obtained by Kolbes decarboxylation of B, C is ![]()
![]()
![]() .
.
Therefore A = Pentan -3 one, ![]()
B = Propanoic acid ![]()
And C = Butane ![]()
The sequence of reactions is
![]()
![]()
Ans 2.
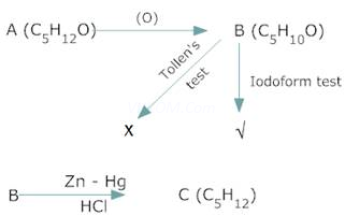
Since B gives a negative Tollen’s test but positive Iodoform test, it is methyl ketone, i.e,![]() . Also it is formed by oxidation of A.
. Also it is formed by oxidation of A.
Therefore A is secondary alcohol i.e, ![]() on reduction B gives pentane with Zn –Hg/ HCl.
on reduction B gives pentane with Zn –Hg/ HCl.
Therefore C is ![]()
Therefore
A = ![]()
B = ![]()
C= ![]()
Reactions:-
Ans 3.
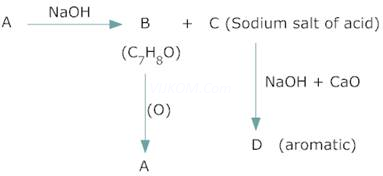
The molecular formula of (B) and characteristic odour of (A) suggests that (A) is an aromatic aldehyde, ![]() and (B) is alcohol,
and (B) is alcohol,![]() . As (C) is a sodium salt of an acid & gives hydrocarbon (D) on heating with soda lime, (C) is sodium benzoate and (D) is benzene.
. As (C) is a sodium salt of an acid & gives hydrocarbon (D) on heating with soda lime, (C) is sodium benzoate and (D) is benzene.
Therefore:-
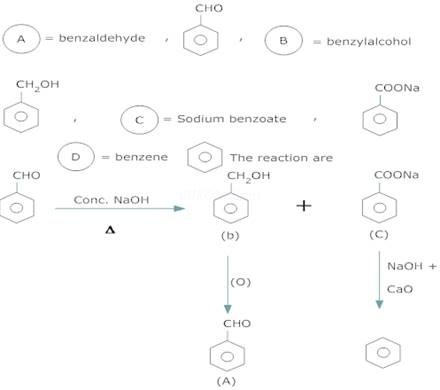
Ans 4.
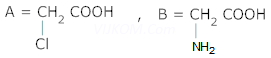
Ans 5. (A) = ![]()
(B) = 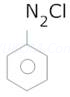
(C) = ![]()
Ans 6. (A) = ![]()
(B) = ![]()
(C) = ![]()
Ans 7. (A) = ![]()
(B) = ![]()
(C) = ![]()
Ans 8. X = 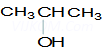
Y= 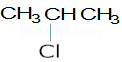
Z = ![]()
Assertion and Reason Answers-
1. (c) Assertion is correct statement but reason is wrong statement.
Explanation:
Aromatic aldehydes and formaldehyde do not contain α-hydrogen and thus undergo Cannizzaro reaction. Formaldehyde is more reactive than aromatic aldehydes.
2. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
Explanation:
O-Substituted benzoic acids are generally stronger acids than benzoic acid. This is regardless of the nature(+I or -I) of the substituent. This is called ortho-effect and is probably due to a combination of steric and electronic factors.
Case Study Answers-
1. Answer :
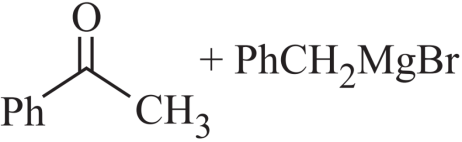
Explanation:
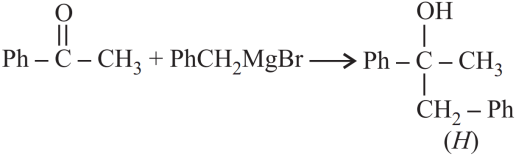
Explanation:
Explanation:
Explanation:
2. Answer :
Explanation:
It is an example of cross Cannizzaro reaction where aromatic aldehyde gets reduced to alcohol and aliphatic aldehyde gets oxidised to its sodium salt (both aldehydes must not contain any ∝−∝−hydrogen).
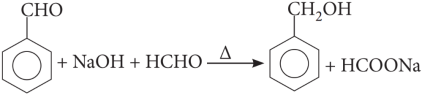
Explanation:
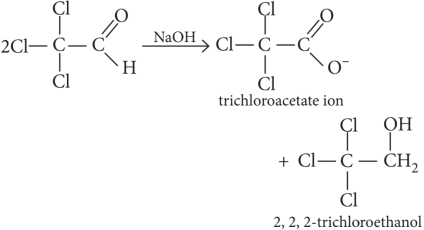
The Cannizzaro product of given reaction yields 2, 2, 2-trichloroethanol.
Explanation:
Hydride transfer is the slowest step.
Explanation:
C-C bond is not formed in Cannizzaro reaction while other reactions result in the formation of C-C bond.