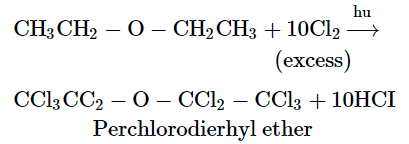Alcohols, Phenols, and Ethers
For example:C2H5-O-C2H5(Dimethylether)
Nomenclature
Alcohols
Incaseof cycliccompounds,weusetheprefixcycloifthe–OHgroupisattachedtoC-1.
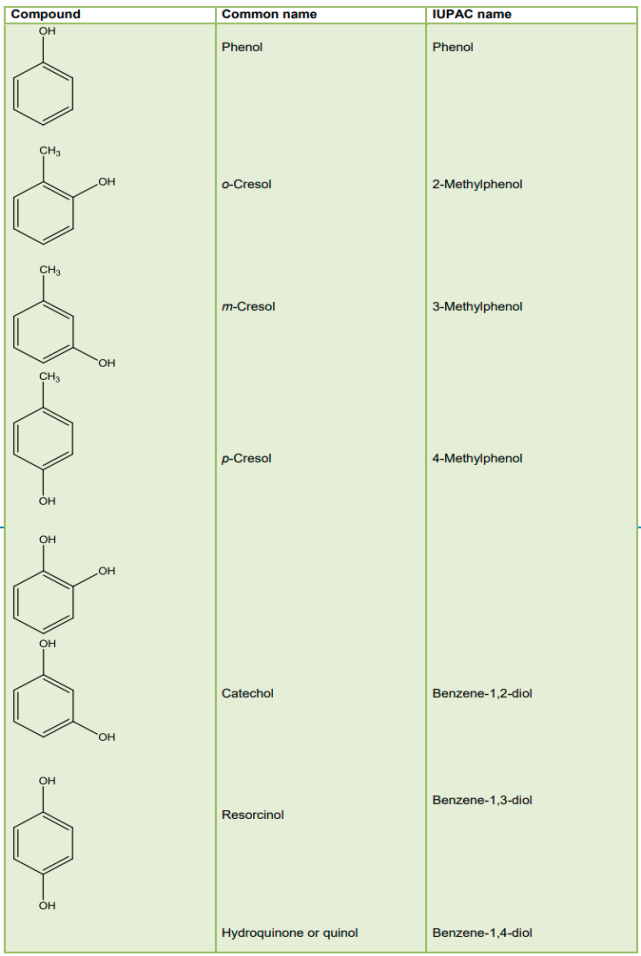
Phenols:
Ethers

StructuresofFunctionalGroups
Alcohols
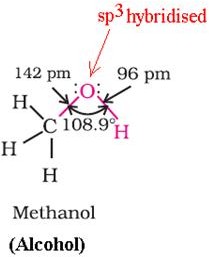 The bond is formed by the overlap of sp3hybridised orbital of carbon with a sp3hybridised orbital ofoxygen.
The bond is formed by the overlap of sp3hybridised orbital of carbon with a sp3hybridised orbital ofoxygen.
Phenols
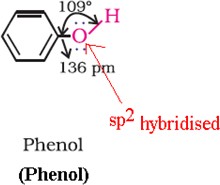
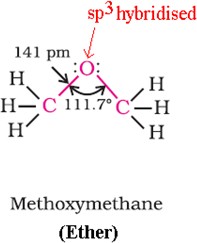
Ethers
PreparationofAlcohols
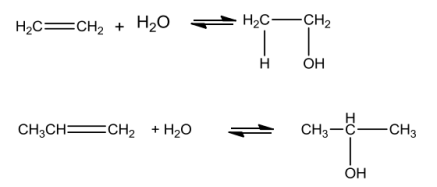
FromAlkenes
Alcoholsarepreparedbytreatingalkeneswith waterinthepresenceofacidascatalyst.
Alkenesontreatmentwithdiboranegivetrialkyl boranesasadditionproductwhichisthenoxidisedtoalcoholbyhydrogenperoxideinthepresenceofaqueous sodiumhydroxide.
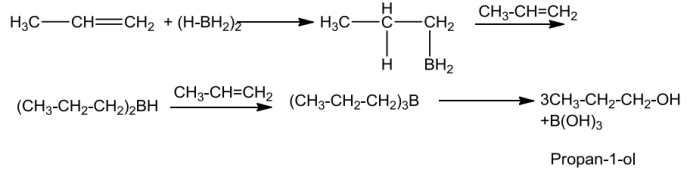
Theadditionofboranetothedoublebondtakesplaceinsuchawaythattheborongetsaddedtothesp2carbonwithmorenumber ofhydrogenatoms.
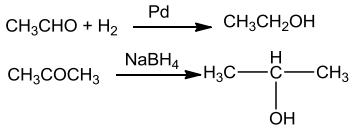
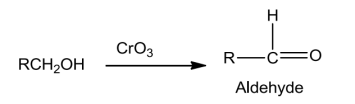
FromCarbonylCompounds
![]()
![]()
![]()
![]()
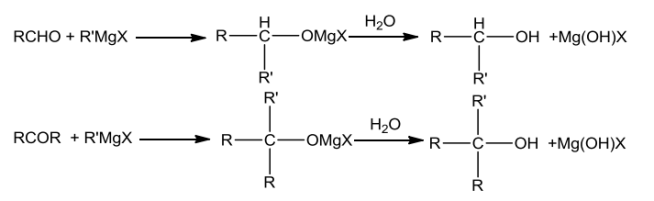
FromGrignardreagents
Grignardreagentsonreactingwithaldehydesandketonesyieldalcohols.
PreparationofPhenols
Chlorobenzeneonfusing withNaOHat623Kand320atmosphericpressuregivessodiumphenoxidewhichonacidificationyields phenol.
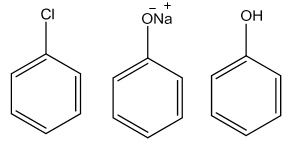
![]()
![]()
![]()
![]()
![]()
Benzeneonsulphonationwitholeumgivesbenzenesulphonicacidwhichonheatingwithmoltensodiumhydroxidegives sodiumphenoxide.Acidificationofthesodiumphenoxidegivesphenol.
![]()
![]()
![]()
![]()
![]()
![]()
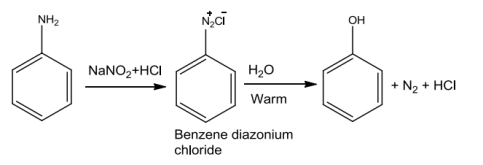
Anilineontreatment withnitrousacidat 273-278Kgivesbenzenediazoniumchloride whichonhydrolysiswithwarmwaterortreatment withdiluteacidsisconvertedtophenols.
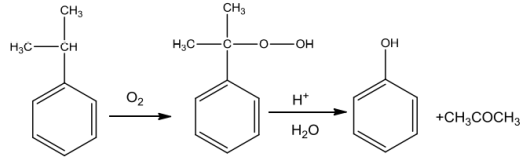
Cumene(isopropylbenzene)onoxidation withairgivescumenehydroperoxidewhichontreatment withdiluteacidis convertedtophenol.
PhysicalProperties
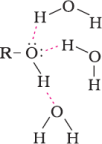
ChemicalProperties
Alcoholsreactbothasnucleophilesandelectrophiles.
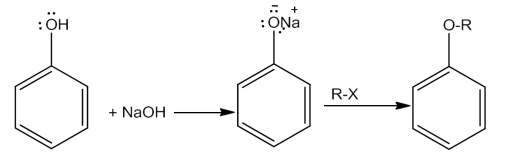
Alcohols and phenols react with active metals like Na, K and Al to give correspondingalkoxides/phenoxideswiththeevolutionofhydrogen.
2R-OH+ 2Na→ 2R-O-Na+ H2
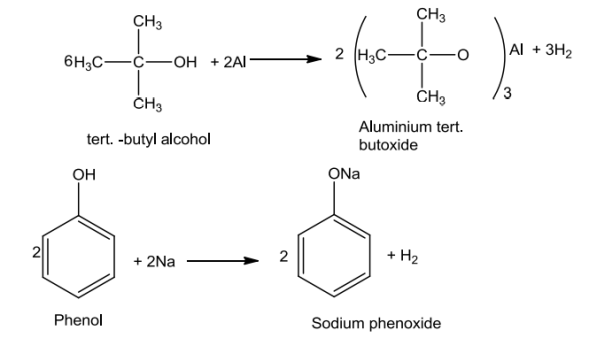
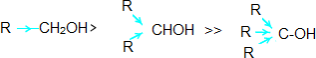
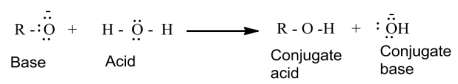
Inthereaction,waterisabetterprotondonor(i.e.,strongeracid)thanalcohol.Overherethe alkoxide ion is a better proton acceptor than hydroxide ion which suggests thatalkoxidesarestronger bases.
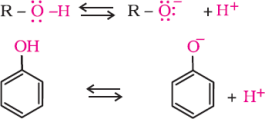
![]()
![]()
![]()
![]()

![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
Only alcohols show reactions involving cleavage of C-O bond. Phenols exhibit this type of reactiononlywithzinc.
Alcohols on treatment with hydrogen halides form alkyl halides.
ROH+ HX →R-X +H2O
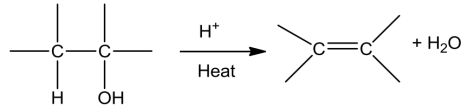
HowtodistinguishbetweenPrimary,SecondaryandTertiaryAlcohols?
Lucasreagenttest
![]()
Alcohols get converted into alkyl bromides on treatment with PBr3.
3R-OH+ PBr3→ 3R-Br+ H3PO3
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()

![]()
![]()
![]()
![]()
![]() Tertiary> Secondary> Primary
Tertiary> Secondary> Primary
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
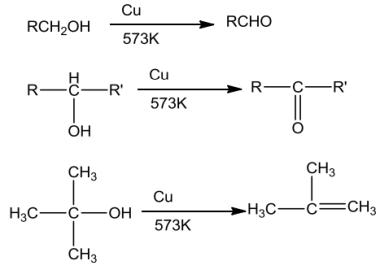
CharacteristicsofPhenols
Phenolontreatment withdil.HNO3at lowtemperatureyieldsamixtureoforthoandpara nitrophenols.
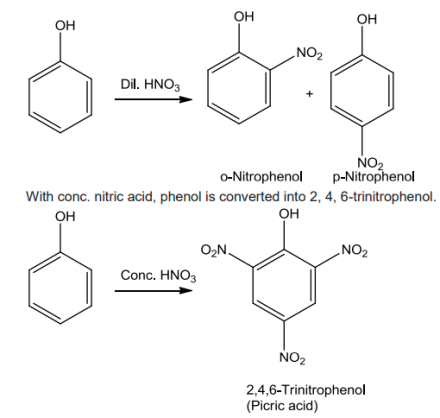
Monobromophenols are formed when phenol is treated with bromine in CHCl3 or CS2 at lowtemperature.
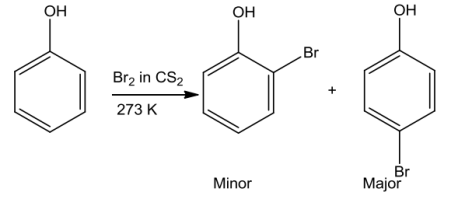
Ontreatingphenolwithbrominewater, awhiteprecipitateof 2, 4,6-tribromophenolisformed.
Phenols on treatment with NaOH produces phenoxide ion which is even more reactive thanphenol towards electrophilic aromatic substitution and therefore it undergoes electrophilicsubstitutionwithcarbondioxide.Orthohydroxybenzoicacidisobtained asthemainproduct.
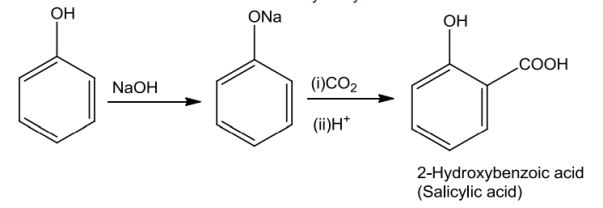
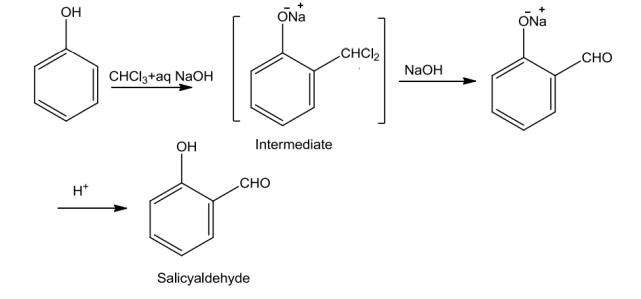
PhenolsontreatmentwithchloroforminthepresenceofNaOH,a–CHOgroupisintroducedatortho position of benzene ring. The substituted benzal chloride formed as intermediate onhydrolysiswithalkaliproducesalicylaldehyde.
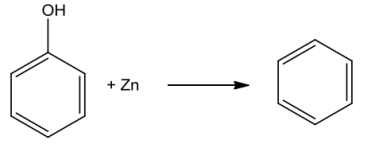
Phenolonheating withzincdustproducesbenzene.
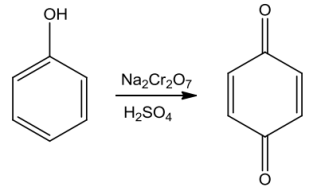
Phenolsonoxidation withchromicacidgivesoutconjugateddiketone knownasbenzoquinone.
SomeCommerciallyImportantAlcohols
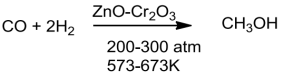
Methanolandethanolaretwocommerciallyimportantalcohols.
Methanol
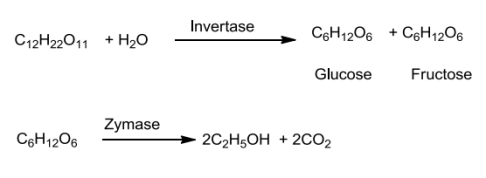
Ethanol
PreparationofEthers
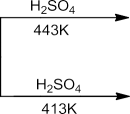
![]()
![]()
PhysicalPropertiesofEthers
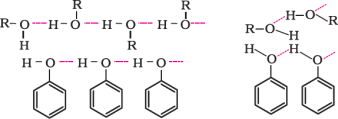
Miscibility of ethers with water resembles those of alcohols of the same molecular mass. This isbecausesimilartoalcohols;oxygenofethercanalsoform hydrogenbondswiththe watermolecule.
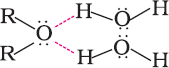
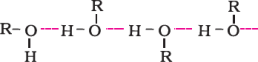 Ethershavemuchlowerboilingpointsthanalcohols.Thisisduetothepresenceofhydrogenbondinginalcohols.Hydrogenbondingis absentinethers.
Ethershavemuchlowerboilingpointsthanalcohols.Thisisduetothepresenceofhydrogenbondinginalcohols.Hydrogenbondingis absentinethers.
ChemicalPropertiesofEthers
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
Thealkoxygroup(-OR)isortho,paradirecting andactivatesthebenzeneringforaromatic substitution.
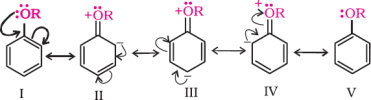
![]()
![]()
![]()
![]()
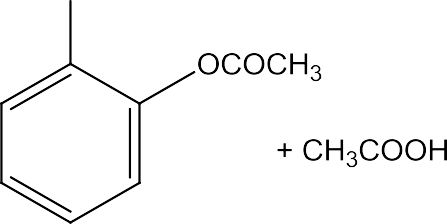
![]() Phenylalkylethersundergohalogenationreaction.
Phenylalkylethersundergohalogenationreaction.
Inthisreactionthealkylgroupsandacylgroupsareintroducedatorthoandparapositionbytreatinganisolewithalkylhalideandacylhalideinthepresenceofanhydrouschlorideascatalyst.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
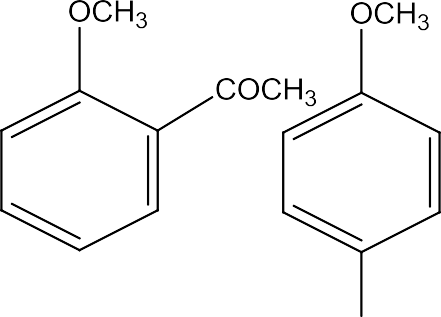
![]()


Anisoleontreating withamixtureofsulphuricacidandnitricgivesamixtureoforthoandparanitroanisole.
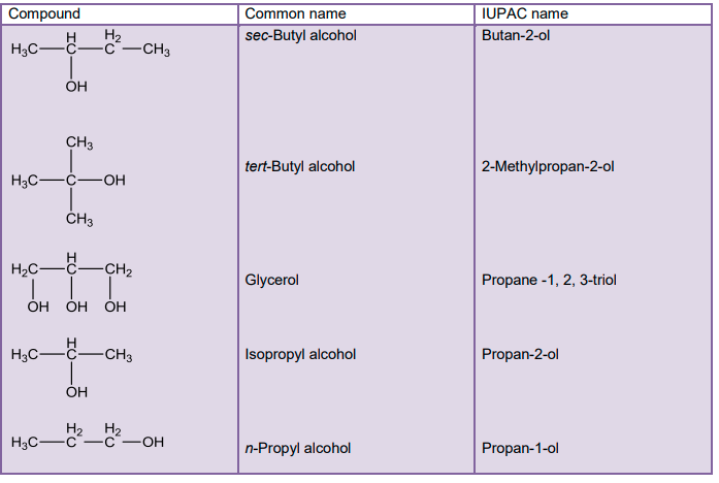
Classification of alcohols
We can classify alcohols on the basis of different factors:
Number of hydroxyl groups attached , hybridization ,number of alkyl groups attached to alpha carbon (alpha carbon is that which has functional groups attached to it )
On the basis of number of –OH(hydroxyl ) group attached we have :
Monohydric alcohols: They are those that have only one hydroxyl group attached.
Example: methanol (CH3OH) ethanol (C2H5OH) and more
Dihydric alcohols: They have two hydroxyl groups attached.
Example: ethylene-glycol
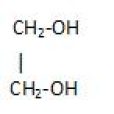
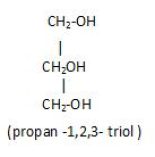
Polyhydric alcohol: That have three or more hydroxyl groups in it.
Example glycerol
(2). Based on the type of hybridization:
It can be sp3 like ethanol – CH3-CH2-OH
Another example :
It can be sp2 like Benzyl alcohol-
In this second carbon is alpha carbon and that is sp3
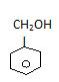
(Benzyl alcohol)
Allyl alcohol CH2=CH- CH2OH (In it also alpha carbon is sp3hybridised ).
Another type of hybridization they possess is sp2
Example: PhenolC6H5OH, Vinyl alcohol CH3-CH2 =CH-OH etc
(c) Classification is on the basis of primary secondary or tertiary carbon atom (alpha carbon)
SSS
10 2 30
Important Questions
Multiple Choice questions-
Question 1.Among the following compounds, strongest acid is
(a) H-C = C-H
(b) C6H6
(c) C2H6
(d) CH3OH
Question 2.1-Propanol and 2-propanol can be best distinguished by
(a) Oxidation with KMnO4 followed by reaction with Fehling solution?
(b) Oxidation with acidic dichromate followed by reaction with Fehling solution.
(c) Oxidation by heating with copper followed by reaction with Fehling solution.
(d) Oxidation with cone. H2SO4 followed by reaction with Fehling solution.
Question 3.The compound which gives the most stable carbonium ion on dehydration is
(a) (CH3)2CHCH2OH
(b) (CH3)3COH
(c) CH3CH2CH2CH2OH
(d) CH3CH OH CH2 CH3
Question 4.In the following compounds:
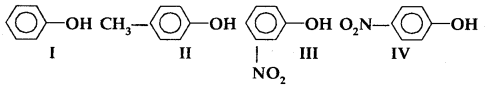
The order of acidity is
(a) III > IV > I > II
(b) I > IV > III > II
(c) II > I > III > IV
(d) IV > III > I > II
Question 5.In CH3 CH2 OH, the bond that undergoes heterolytical change most readily is
(a) C-C
(b) C-O
(c) C-H
(d) O-H
Question 6.Phenol reacts with Br2 in CS2 at low temperature to give
(a) o-Bromophenol
(b) o-and p-promophenols
(c) p-Bromophenol
(d) 2, 4, 6Tribromophenol
Question 7.In the reaction of phenol with CHCl3 and aqueous NaOH at 343 K, the electrophile attacking the ring is:
(a) CHCl3
(b) CHCl2
(c) CCl2
(d) COCl2
Question 8.Which of the following is most acidic?
(a) Phenol
(b) Benzyl alcohol
(c) m-chlorophenol
(d) cyclohexanol
Question 9.The correct order of boiling points for primary (1°), Secondary (2°) and Tertiery (3°) alcohols is
(a) 1° > 2° > 3°
(b) 3° > 2° > 1°
(c) 2° > 1° > 3°
(d) 2° > 3° > 1°
Question 10.When Phenol is distilled with zinc dust, it gives
(a) Benzene
(b) Toluene
(c) Benzaldehyde
(d) Benzoic acid
Very Short Questions–
1. Write IUPAC names of :–
i. 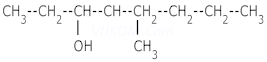
ii. 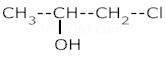
iii. 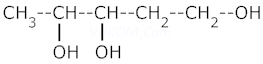
iv. ![]()
v. 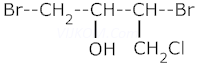
vi. 
vii. 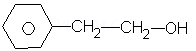
viii. 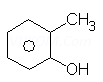
ix. 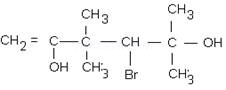
x. 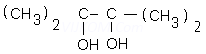
Short Questions–
1. Phenol is acidic in nature.
2. Phenol has a smaller dipole moment than methanol.
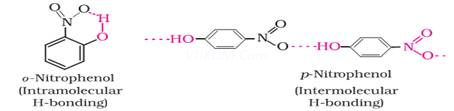
3. o- nitrophenol has lower boiling point (is more volatile) than p – nitrophenol.
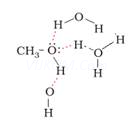
4. Methanol is miscible with water while iodomethane is not.
5. Alcohols have higher boiling points than isomeric ethers.
6. Ethers are soluble in water alkanes are not.
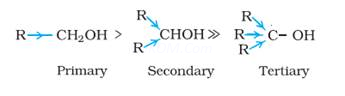
7. The order of acidic strength in alcohols is R ![]()
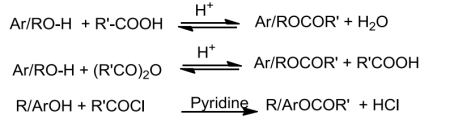
8. During preparation of ester from alcohol and acid, water has to be removed as soon as it is formed.
9. Ethers can not be prepared by dehydration of secondary or tertiary alcohols.
10. Reaction of anisole with HI gives methyl iodide and phenol.
Long Questions–
1. Classify the following as primary, secondary and tertiary alcohols:
(i)
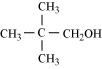
(ii) ![]()
(iii) ![]()
(iv)
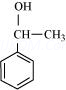
(v)
![]()
(vi)
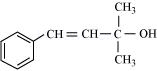
2. Name the following compounds according to IUPAC system.
(i)
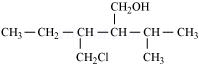
(ii)
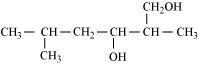
(iii)
(iv)
![]()
(v)
![]()
3. Give structures of the products you would expect when each of the following alcohol reacts with (a) ![]() (b) HBr and (c)
(b) HBr and (c) ![]() .
.
(i) Butan-1-ol
(ii) 2-Methylbutan-2-ol
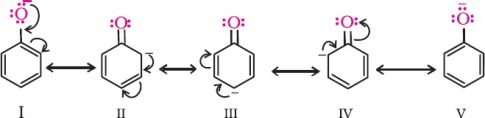
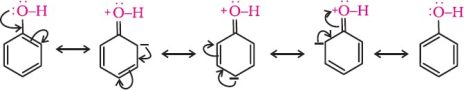
4. Ortho and para nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide ions.
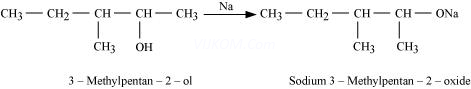
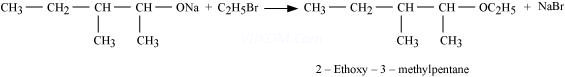
5. Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3-methylpentan-2-ol.
6. Predict the products of the following reactions:
(i) ![]()
(ii)
![]()
(iii)
![]()
(iv) ![]()
7. Write IUPAC names of the following compounds:
(i)
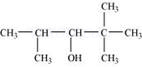
(ii)
![]()
(iii)
![]()
(iv)
![]()
(v)
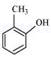
(vi)
![]()
(vii)
![]()
(viii)
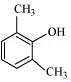
(ix)
![]()
(x) ![]()
(xi) ![]()
(xii)
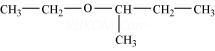
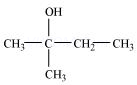
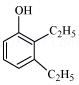
8. Write structures of the compounds whose IUPAC names are as follows:
(i) 2-Methylbutan-2-ol
(ii) 1-Phenylpropan-2-ol
(iii) 3,5-Dimethylhexane -1, 3, 5-triol
(iv) 2,3 – Diethylphenol
(v) 1 – Ethoxypropane
(vi) 2-Ethoxy-3-methylpentane
(vii) Cyclohexylmethanol
(viii) 3-Cyclohexylpentan-3-ol
(ix) Cyclopent-3-en-1-ol
(x) 3-Chloromethylpentan-1-ol.
Assertion and Reason Questions–
1.In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Primary and secondary alcohols can be distinguished by Victor-Meyer’s test.
Reason: Primary alcohols form nitrolic acid which dissolves in NaOH to form blood red colouration but secondary alcohols form pseudonitrols which give blue colouration with NaOH.
2.In these questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
Assertion: Reimer-Tiemann reaction of phenol with CHCl3 in Na OH at 340K gives salicylic acid as the major product.
Reason: The reaction occurs through intermediate formation of +CHCl2.
Case Study Questions–
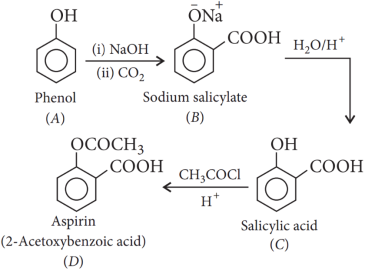
1.Read the passage given below and answer the following questions:
An organic compound (A) having molecular formula C6H6O gives a characteristic colour with aqueous FeCl3 solution. (A) on treatment with CO2 and NaOH at 400K under pressure gives (B), which on acidification gives a compound (C). The compound (C) reacts with acetyl chloride to give (D) which is a popular pain killer.
The following questions are multiple choice questions. Choose the most appropriate answer:
2. Read the passage given below and answer the following questions:
A compound (X) containing C, H and O is unreactive towards sodium. It also does not react with Schiff s reagent. On refluxing with an excess of hydroiodic acid, (X) yields only one organic product ( Y). On hydrolysis, (Y) yields a new compound (Z) which can be converted into (Y) by reaction with red phosphorus and iodine. The compound (Z) on oxidation with potassium permanganate gives a carboxylic acid. The equivalent weight of this acid is 60.
The following questions are multiple choice questions. Choose the most appropriate answer:
MCQ Answers–
Very Short Answers–
Short Answers–
Ans 1. Phenol is acidic in nature because
(a) phenol, due to resonance, the positive charge rests on oxygen making the shared pair of electrons more towards oxygen and hydrogen as ![]()
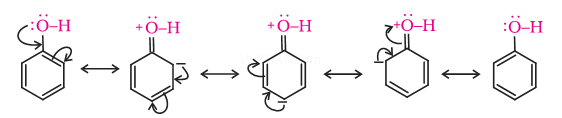
(b) The carbon attached to OH is ![]() hybridize and is more electronegative, this decreases the electron density on oxygen, increasing the polarity of O-H bond and ionization of phenol.
hybridize and is more electronegative, this decreases the electron density on oxygen, increasing the polarity of O-H bond and ionization of phenol.
The phenoxide ion formed by loss of ![]() is more resonance stabilized than phenol itself.
is more resonance stabilized than phenol itself.
Ans 2. In phenol due to electron rich benzene ring the C-O bond is less polar whereas in methanol the C-O bond is highly polar. Therefore the dipole moment of methanol is higher than phenol.
Ans 3. P- nitrophenol has intermolecular hydrogen bonding which increases the boiling point while in o- nitro phenol due to presence of intra molecular hydrogen bonding, there is a decrease in boiling point and increase in volatility.
Ans 4. Methanol can form intermolecular hydrogen bonding with water but there is no hydrogen bonding in iodomethane and water. Therefore methanol in miscible in water.
Ans 5. Alcohols can form intermolecular hydrogen bonds due to their high polarity whereas, ether cannot. Therefore alcohols have higher boiling points than isomeric ethers.
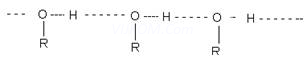
Ans 6. Ethers can form H- bonding with water molecule whereas alkenes cannot. Therefore ethers are soluble in water and alkanes are not.

Ans 7. In alcohols, the acidic strength is due to polar nature of O-H bond. An electron releasing group e.g., alkyl groups, increases electron density on oxygen tending to decrease the polarity of O-H bond. This decreases the acid strength. Therefore the order of acid strength is .
Ans 8. The reaction between alcohol and carboxylic acid is reversible and goes in backward direction if water is not removed as soon as it is formed.
![]()
Ans 9. For secondary and tertiary alcohols, elimination competes over substitution and alkenes are formed on acidic dehydration as the reaction follows Sn1 mechanism. Therefore the acidic dehydration of secondary or tertiary alcohols does not give ethers.
Ans 10. In case of anisole, methyl phenyl oxonium ion, ![]() is formed by protonation of ethers during reaction with HI. The bond between O- CH3 is weaker than the bond between
is formed by protonation of ethers during reaction with HI. The bond between O- CH3 is weaker than the bond between ![]() because carbon of phenyl group is
because carbon of phenyl group is ![]() hybridised and there is a partial double bond character. Therefore the attack by I– ion breaks
hybridised and there is a partial double bond character. Therefore the attack by I– ion breaks ![]() bond to form CH3I.
bond to form CH3I.
![]()
![]()
Long Answers–
Ans 1. Primary alcohol → (i), (ii), (iii)
Secondary alcohol → (iv), (v)
Tertiary alcohol → (vi)
Ans 2. (i) 3-Chloromethyl-2-isopropylpentan-1-ol
(ii) 2, 5-Dimethylhexane-1, 3-diol
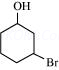
(iii) 3-Bromocyclohexanol
(iv) Hex-1-en-3-ol
(v) 2-Bromo-3-methylbut-2-en-1-ol
Ans 3. (a)(i)
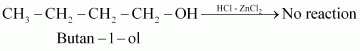
Primary alcohols do not react appreciably with Lucas’ reagent (HCl-ZnCl2) at room temperature.
(ii)
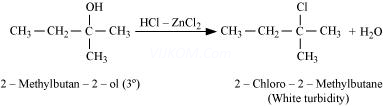
Tertiary alcohols react immediately with Lucas’ reagent.
(b)
(i)
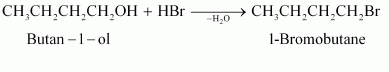
(ii)
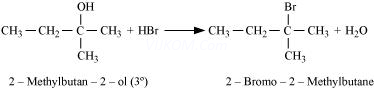
(c)
(i)
![]()
(ii)
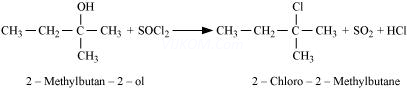
Ans 4.
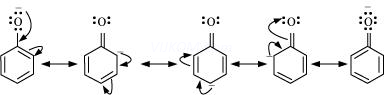
Resonance structure of the phenoxide ion
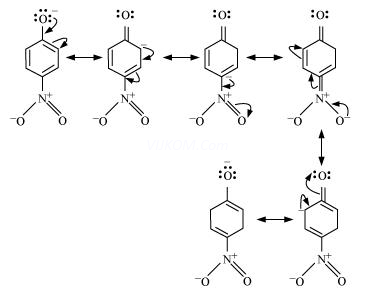
Resonance structures of p–nitrophenoxide ion
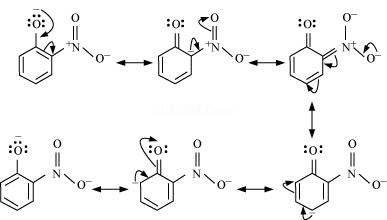
Resonance structures of o–nitrophenoxide ion
It can be observed that the presence of nitro groups increases the stability of phenoxide ion.
Ans 5. In Williamson synthesis, an alkyl halide reacts with an alkoxide ion. Also, it is an ![]() reaction. In the reaction, alkyl halides should be primary having the least steric hindrance. Hence, an alkyl halide is obtained from ethanol and alkoxide ion from 3-methylpentan-2-ol.
reaction. In the reaction, alkyl halides should be primary having the least steric hindrance. Hence, an alkyl halide is obtained from ethanol and alkoxide ion from 3-methylpentan-2-ol.
![]()
Ans 6. (i)
(ii)
(iii)

(iv)
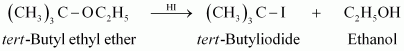
Ans 7. (i) 2, 2, 4-Trimethylpentan-3-ol
(ii) 5-Ethylheptane-2, 4-diol
(iii) Butane-2, 3-diol
(iv) Propane-1, 2, 3-triol
(v) 2-Methylphenol
(vi) 4-Methylphenol
(vii) 2, 5-Dimethylphenol
(viii) 2, 6-Dimethylphenol
(ix) 1-Methoxy-2-methylpropane
(x) Ethoxybenzene
(xi) 1-Phenoxyheptane
(xii) 2-Ethoxybutane
Ans 8. (i)
(ii)
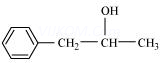
(iii)

(iv)
(v)
![]()
(vi)
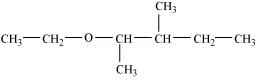
(vii)
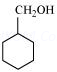
(viii)
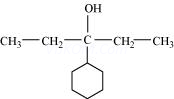
(ix)
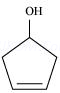
(x)
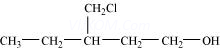
Assertion and Reason Answers–
1. (a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
2. (c) Assertion is correct statement but reason is wrong statement.
Explanation:
Intermediate formed is dichlorocarbene.
Case Study Answers–
1. Answer :
Explanation:
Explanation:
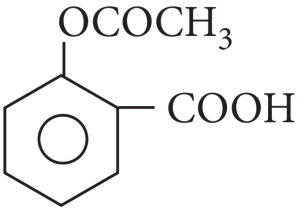
It has 9 C-atoms.
Explanation:
Sodium phenoxide when heated with CO2 at 400K under a pressure of 4-7 atm followed by acidification gives 2-hydroxybenzoic acid (salicylic acid) as the main product along with a small amount of 4-hydroxybenzoic acid. This reaction is called Kolbe’s reaction.
Explanation:
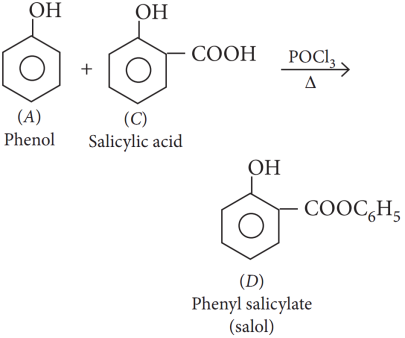
Salol is used as an intestinal antiseptic.
2. Answer :
Explanation:
Since the compound X is unreactive towards sodium so it is neither an acid nor an alcohol. Since the compound X is unreactive towards Schiff’s base so it is not an aldehyde.
The compound X forms only one product on reaction with excess HI, indicates that the compound X may be ether.
Explanation:
The reactions can be written as:
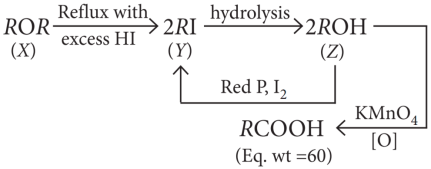
Since the equivalent weight of carboxylic acid is 60. So, it must be CH3COOH i.e., ethanoic acid.
Explanation:
The alcohol Z in that case should be C2H5OH and the compound Y should be ethyl iodide. X is therefore diethyl ether (C2H5 — O — C2H5)
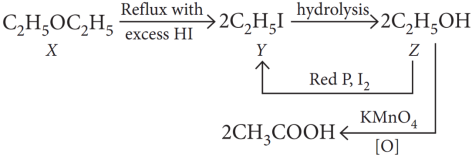
Explanation:
In the presence of light and excess of chlorine, all the hydrogen atoms of diethyl ether are substituted to give perchlorodiethyl ether.